Background
Ischemic stroke is a leading cause of mortality and preventable disability.Reference Katan and Luft1,2 The treatment of acute debilitating large vessel occlusion ischemic stroke has been revolutionized in the wake of multiple randomized controlled trials that show superior clinical and radiologic outcomes with endovascular thrombectomy (EVT) compared to medical management including IV thrombolysis.Reference Saver, Goyal and Bonafe3-Reference Berkhemer, Fransen and Beumer7 These trials had stringent inclusion and exclusion criteria, but given the uniformity of the outcomes, with low numbers need to treat in the 2–4 range, it is important to determine whether additional subgroups of patients excluded from the original trials might also benefit. Emerging evidence supports expanded treatment window to at least 24 hours, increasing the number of stroke patients that may benefit from EVT.Reference Nogueira, Jadhav and Haussen8 However, Grade 1A recommendations in the current guidelines still reserve the use of mechanical thrombectomy for ischemic stroke patients with Alberta Stroke Program Early Computed Tomography Score (ASPECTS) of >5, in an attempt to exclude patients with “large core” established infarcts which were assumed to be unlikely to benefit.Reference Powers, Rabinstein and Ackerson9 Reperfusion of large infarcts is thought to be potentially futile and may increase risk for symptomatic intracranial hemorrhage (sICH).Reference Horsch, Dankbaar and van der Graaf10
However, recent pooled data offered by the Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration investigating EVT for large vessel occlusion ischemic stroke revealed a consistent benefit associated with the intervention across all infarct core volumes, including those considered to be large core infarcts (≥70 cc). These benefits were especially apparent in young patients.Reference Campbell, Majoie and Albers11 In a separate meta-analysis that included additional trials, the HERMES collaboration highlighted an exploratory analysis which showed the benefit of EVT even in patients with ASPECTS between 3 and 5, but not for those with very low ASPECTS (0–2).Reference Román, Menon and Blasco12 The analysis also showed an increased sICH rate of 19% for low ASPECTS (<6) patients who underwent mechanical thrombectomy, which is more than four times the 4.4% sICH rate found across all ASPECTS.Reference Román, Menon and Blasco12,Reference Goyal, Menon and van Zwam13 As studies emerging from the HERMES collaboration were based on trialsReference Saver, Goyal and Bonafe3-Reference Goyal, Demchuk and Menon5 that sought to exclude those with large core infarcts, the numbers for this subgroup of patients with low ASPECTS is understandably low.
With a number needed to treat of only 2.6 for a reduction in 1 modified Rankin Scale (mRS) point,Reference Goyal, Menon and van Zwam13 EVT has definitively transformed the management of large vessel occlusion ischemic stroke in carefully selected patients. However, if the recommended American Heart Association Stroke guidelinesReference Powers, Rabinstein and Ackerson9 for EVT were to be strictly applied in clinical practice, a substantial number of patients, including the subset of patients with low ASPECTS, would be left with the dismal natural history of large vessel occlusion stroke with 73.5%Reference Goyal, Menon and van Zwam13 of patients being dead or dependent (mRS of 3–6) at 90 days.
Data derived from retrospective observational studies from EVT registries that operate outside the strict confines of a trial can provide insight regarding the outcomes in this subset of patients who undergo EVT. Several observational studiesReference Logan, Maingard and Phan14-Reference Kim, Yoon, Park, Heo, Baek and Lee17 looked into the outcomes of patients with ASPECTS less than or equal to 6. Their findings unanimously suggest that this subgroup of patients may have benefit comparable to those with higher ASPECTS. Ohta et al.Reference Ohta, Morimoto and Okada18 demonstrated that this benefit is even more apparent when these low ASPECTS patients undergoing EVT are compared to a group who receive medical therapy alone. The strongest evidence comes from a subgroup analysis of a randomized controlled trial (THRACE) that showed a favorable trend (36% vs 26%) toward good (0–2) 90-day mRS in patients who undergo EVT and intravenous thrombolysis compared to those receiving intravenous thrombolysis only for patients with ASPECTS of 0–4.Reference Bracard, Ducrocq and Mas19 Despite the low number (n = 57) of patients with very low ASPECTS in this trial, the results were certainly encouraging.
Through this meta-analysis, we aim to explore the data on the clinical outcomes of patients with low ASPECTS (<6) undergoing EVT, with emphasis on sICH rate, mortality rate and 90-day mRS, mortality rate and sICH rate.
Methods
The guidelines and outlines set by Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA)Reference Moher, Liberati, Tetzlaff and Altman20 and Meta-analyses Of Observational Studies in EpidemiologyReference Stroup21 were adhered to in this systematic review and meta-analysis. Data supporting the results of the study are available from the corresponding author upon reasonable request.
Search Strategy, Information Sources, and Study Selection
MEDLINE, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov were reviewed for pertinent literature published from January 1, 2015 up to April 13, 2019 in line with the PRISMA guidelines. The terms “endovascular” “thrombectomy” “ASPECTS”, and “stroke” were used in various combinations to identify the appropriate studies for the systematic review. The MEDLINE search was done in both MeSH Terms and All Fields. The search on Cochrane was limited to Cochrane Reviews and Trials. On ClinicalTrials.gov, only completed, suspended, and terminated studies were sought. To ensure that all eligible studies were included in the systematic review, authors of studies with incomplete data were contacted by email and hand searching through the references of selected studies was also done.
Study Eligibility
Studies were included based on the following criteria: (1) inclusion of patients who underwent endovascular therapy for acute large vessel occlusion ischemic stroke involving the anterior circulation with an ASPECTS of 5 or less on baseline imaging with cranial computed tomography (CT) or magnetic resonance imaging (MRI) scan; (2) description of 90-day mRS outcomes and sICH, (3) written in the English language. Studies with small sample size (<10), involving the pediatric population and conference abstracts, were excluded from the review.
Risk of Bias
All the studies included were observational studies. Five studies were single-group cohorts that only included patients undergoing EVT and therefore deemed to have high risk of bias. Nonetheless, the quality of these studies was assessed using an evaluation tool for case seriesReference Murad, Sultan, Haffar and Bazerbachi22 (see Table 1 in Supplemental Digital Content). The remaining four studies with data for both EVT and best medical therapy arms were rated according to the Newcastle–Ottawa Assessment Scale for Cohort StudiesReference Wells, Shea and Connell23 (see Table 2 in Supplemental Digital Content). These five studies had low degree of bias.
Data Collection Process and Data Items
Two physicians (JBD and KP) independently reviewed the selected studies for quality and extracted the following data from the full text articles: title, author, study period, year of publication, number of patients, patient characteristics (age, sex), ASPECTS range, 90-day mRS, and sICH. We requested for additional details by emailing the authors for studies with incomplete data. All data were tabulated in a spreadsheet (Microsoft Excel for Mac v16.28) for analysis. Any disagreements with data extraction and article appraisal were settled by a third independent reviewer (AAD). If the study did not provide means and standard deviations, we estimated these means and standard deviations from the median and range values if these data were not available.Reference Hozo, Djulbegovic and Hozo24 The primary outcomes for this study were 90-day mRS scores, mortality, and the rate of sICH. Data on patients who underwent best medical therapy were included. The best medical therapy group includes large vessel occlusion patients who received standard of care stroke management which include but are not limited to anticoagulation, antiplatelet therapy, and thrombolysis.
Statistical analysis and Synthesis of Results
A meta-analysis of proportions was conducted for the available main perioperative and postoperative variables. Firstly, to establish variance of raw proportions, a logit transformation was applied. To incorporate heterogeneity (anticipated among the included studies), transformed proportions were combined using DerSimonian–Laird random effects models. Finally, the pooled estimates were back-transformed. Heterogeneity was evaluated using Cochran’s Q and I2 test. All analyses were performed using the metafor package for R version 3.01 (www.R-project.org). p-Values <0.05 were considered statistically significant.
Results
Study Selection
A total of 635 studies were identified through electronic database searches and other sources. After exclusion of duplicate or irrelevant references, 24 potentially relevant articles were retrieved. After detailed review, nine studies remained for assessment. The flow diagram of the study selection process is presented in Figure 1.
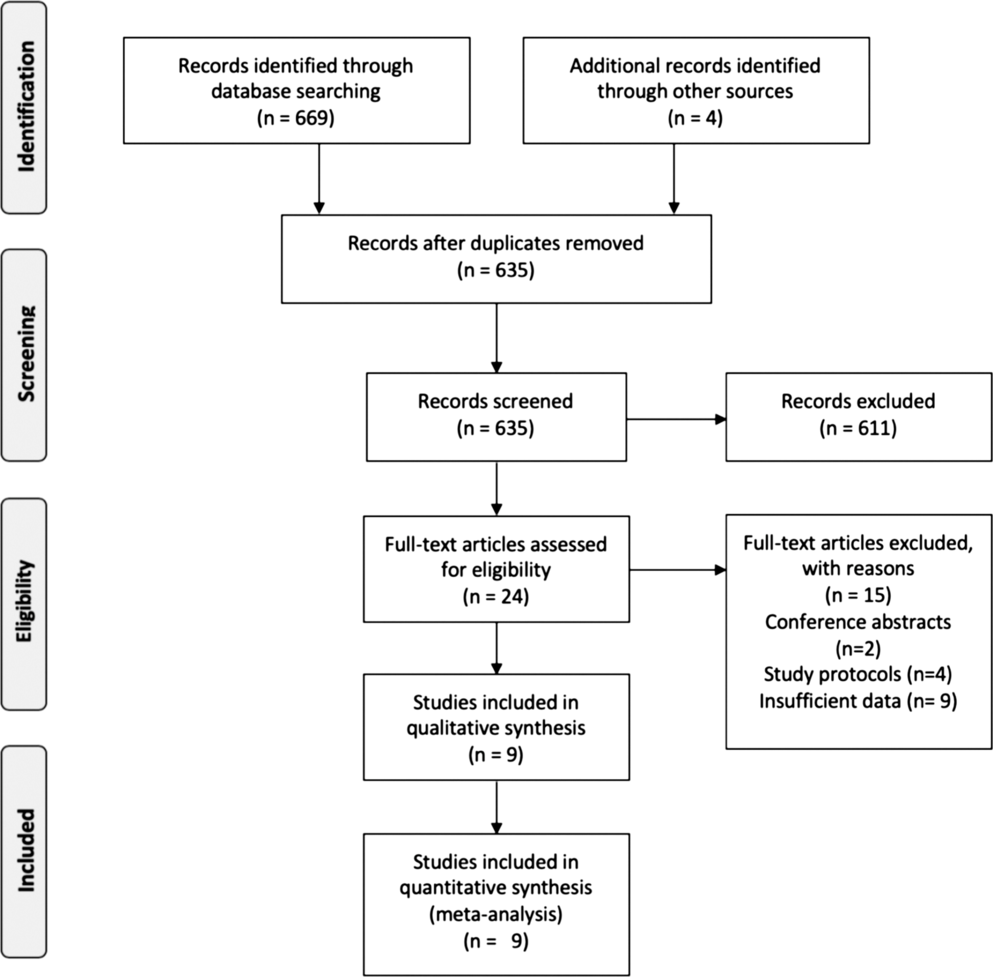
Figure 1: Flow diagram of study selection patients.
Characteristics of Studies and Patients
The nine studies included in the analysis were published from 2016 to 2019. All were observational studies utilizing prospectively collected stroke registry data. Apart from the study by Kakita et al.,Reference Kakita, Yoshimura and Uchida25 all the included studies were retrospective. Four of the nine studies compared low ASPECTS patients undergoing EVT and best medical therapy. The other five studies were observational studies that only looked at outcomes of patients undergoing EVT. A total of 1,196 patients with ASPECTS of 5 or less were included in the analysis. Of these, 712 patients underwent EVT with best medical therapy, while 484 patients only received best medial therapy. The summary of the characteristics of the studies are summarized in Table 1.
Table 1: Characteristics of included studies
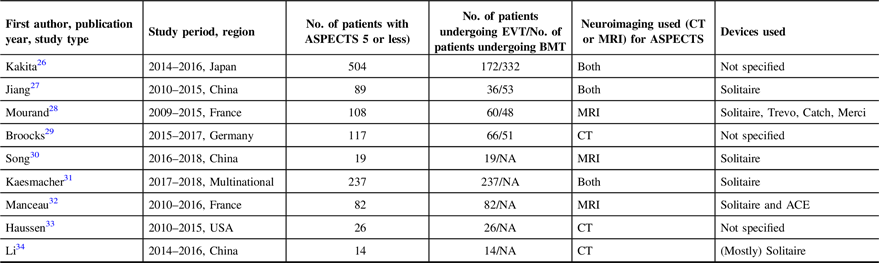
ASPECTS, Alberta Stroke Program Early CT Score; EVT, endovascular thrombectomy; BMT, best medical therapy; CT, computed tomography; MRI, magnetic resonance imaging; NA, not applicable
Both groups are similar in all baseline characteristics, except for more females in the best medical therapy group (49.3% vs 41.8%, p = 0.047) and more diabetic patients in the best medical therapy group (37.3% vs 17.7%, p = 0.007). Of the four studies that indicated the median ASPECTS, two studiesReference Kakita, Yoshimura and Uchida25,Reference Li, Li, Dai, Wang and Xiong34 had significantly lower ASPECTS in the best medical therapy group (ASPECTS 3) compared to the EVT group (ASPECTS 5). There were more distal middle cerebral artery (M2 segment) occlusions in the best medical therapy group compared to the EVT group (15.4% vs 5.4%, p = 0.04). The pooled onset to reperfusion time in the EVT arm was 324 minutes (95% CI 275.949–372.346; I2 92.58%). The baseline characteristics of the patients are summarized in Table 2.
Table 2: Baseline characteristics of patients
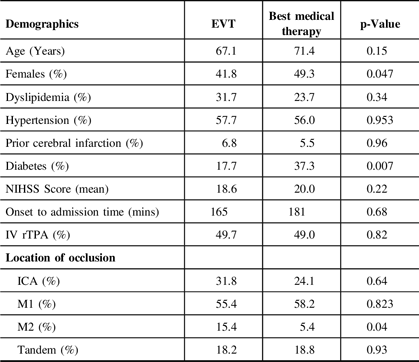
ICA, internal carotid artery; NIHSS, National Institutes of Health Stroke Scale; rTPA, recombinant tissue plasminogen activator.
Symptomatic Hemorrhage, 90-day Mortality, and 90-day Functional Outcomes
Patients in the EVT arm had a pooled recanalization rate of 72.3% (95% CI 61.5–81.0; I2 82.21%). There was a trend toward a higher rate of sICH in the EVT group (9.2%; 95% CI 6.1–13.6; I2 53.37%) compared to the best medical therapy group (5.5%; 95% CI 3.7–8.1; I2 = 0%) but this did not reach statistical significance (p = 0.11) (see Figure 2A). There was no difference (p = 0.41) in the pooled 90-day mortality of EVT patients (30.7%; 95% CI 21.7–41.5; I2 84.23%) and best medical therapy patients (36.6%; 95% CI 26.4–48.1; I2 76.2%) (see Figure 2B). Patients who underwent EVT had significantly better (p = 0.001) 90-day outcomes, with 27.7% (95% CI 21.8–34.5; I2 62.08%) of patients attaining an mRS of 0–2 compared to only 3.7% (95% CI 2.3–5.9; I2 87.21%) of patients in the best medical therapy (see Figure 3). The EVT groups all have moderate to high heterogeneity (I2 > 50%).

Figure 2: Safety of endovascular thrombectomy (EVT) for low ASPECTS patients. (A) Forest plot of pooled proportion of patients with symptomatic intracranial hemorrhage for EVT and best medical management (BMT). (B) Forest plot of pooled proportion of 90-day mortality for endovascular therapy (EVT) and best medical therapy (BMT); The estimate proportion of each trial corresponds to the middle of the squares and the horizontal line shows the 95% confidence interval (CI). For each group, the sum of the statistics, along with the summary proportion, is represented by the middle of the solid diamonds. Weighted data were pooled following logit transformation for proportions. A test of heterogeneity between the trials within a subgroup is also given adjacent to the summary statistics. CI, confidence interval; Evt, events; Trt, treatment sample size.
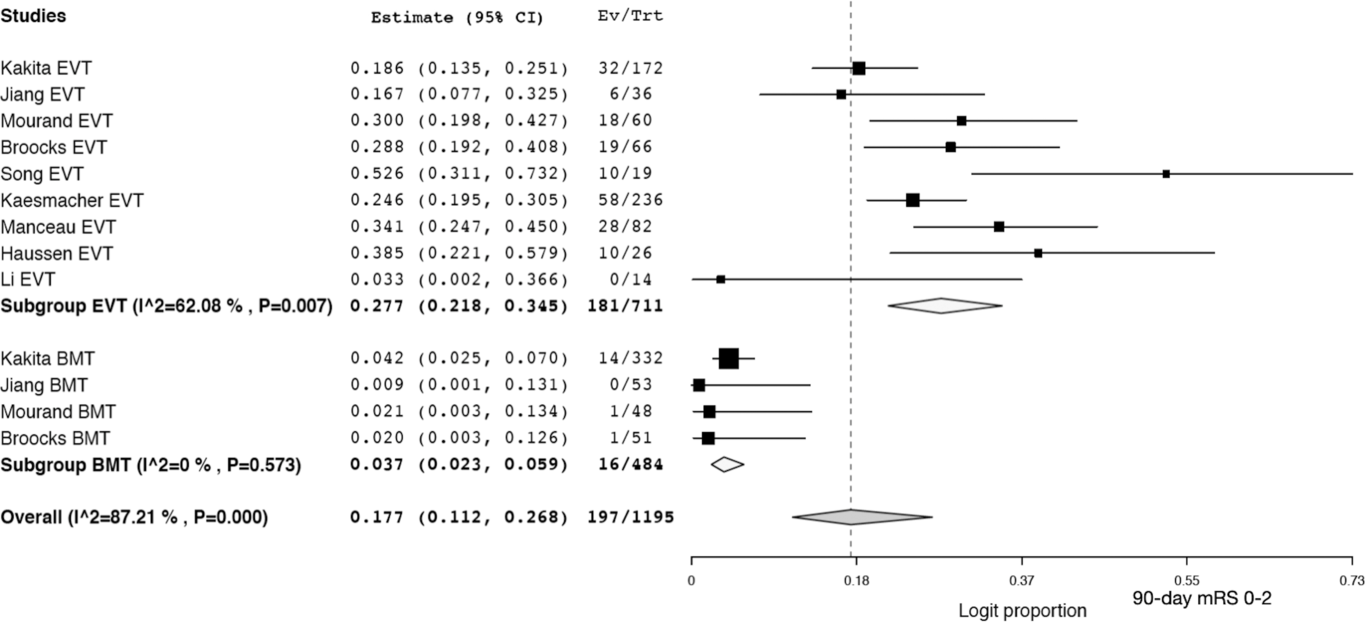
Figure 3: Forest plot of pooled proportion of patients with a 90-day mRS of 0–2 for endovascular therapy (EVT) and best medical therapy (BMT).
Discussion
Summary of Evidence
Our review demonstrates that for patients with low ASPECTS who are considered to have very little remaining salvageable brain tissue, there is a trend toward higher rates of sICH for patients who undergo EVT. Despite this, significantly more patients in the EVT group attain good functional outcomes (mRS of 0–2) at 90 days. However, as we will later discuss, there are important limitations of the study because all the studies included were observational in nature.
Compared to the HERMES collaboration meta-analysisReference Goyal, Menon and van Zwam35 featuring the five landmark trialsReference Saver, Goyal and Bonafe3-Reference Berkhemer, Fransen and Beumer7 for EVT in large vessel occlusion ischemic stroke, our meta-analysis showed higher rates of SICH, mortality, and worse outcomes at 90 days. These are likely because most of the studies included in the HERMES meta-analysis excluded patients with low ASPECTS and/or large ischemic cores.Reference Saver, Goyal and Bonafe3-Reference Goyal, Demchuk and Menon5 Low ASPECTS has been shown to predict intracranial hemorrhage after EVT.Reference Nawabi, Kniep and Schön36,Reference Raychev, Saver and Jahan37 Thus, the difference in sICH rate (9.2% vs 4.4%) is most likely accounted for by the low ASPECTS of our patients (<6) compared to the ones included in the earlier trials (mean ASPECTS of 9).Reference Saver, Goyal and Bonafe3-Reference Goyal, Demchuk and Menon5 The previously reported 19% sICH rate in EVT patients with an ASPECTS of <6 from a subset of the HERMES collaborationReference Román, Menon and Blasco12 was not corroborated by our review that only showed a 9.2% sICH rate. Similar to our findings, an earlier attempt at large vessel reperfusion with the use of intra-arterial thrombolytics (PROACT 2) also showed a sICH rate (10%). Despite this, the patients randomized to the intervention arm still had superior functional outcomes at 90 days.Reference Furlan, Higashida and Wechsler38 The pooled mortality rates in our patients in both the EVT and best medical therapy arms of our review are almost twice that found in the HERMES collaboration. Lower ASPECTS scores implying less salvageable brain and a higher chance of a malignant infarct likely underlie this finding as well.
A previous meta-analysis tackling the effect of ASPECTS on the outcomes of EVT patients suggested futility of the intervention for patients with low ASPECTS.Reference Phan, Saleh and Dmytriw29 What our study reveals is that despite the relatively lower good (mRS of 0–2) 90-day outcomes (27.7%) compared to the earlier trials (46.0%)Reference Goyal, Menon and van Zwam13 utilizing criteria that screened out large core infarct patients, there is still benefit compared to low ASPECTS patients only receiving best medical therapy who have a dismal 3.7% rate of good outcomes at 90 days.
Clinical benefit of EVT for stroke patients with low ASPECTS could for instance be strategic salvage of eloquent areas such as internal capsule and Rolandic cortex. Future directions in the use of ASPECTS for determining EVT eligibility should take into account that not all 10 points of the scoring system that correspond to different middle cerebral artery territories have the same clinical impact. In addition, reduction in net water uptake, a biomarker associated with the development of malignant edema, has also been shown to be decreased in low ASPECTS stroke patients achieving reperfusion after undergoing EVT. This implies that edema reduction may mediate the benefit seen in this subset of patients.Reference Broocks, Hanning and Flottmann39
ASPECTS relies on the ability of the reader to determine early ischemic changes, such as focal swelling and parenchymal hypoattenuation in the territory of the middle cerebral artery.Reference Pexman, Barber and Hill40 It has been shown to have good interobserver reliability in its use for intra-arterial stroke treatment selection.Reference Gupta, Schaefer and Chaudhry41 However, a later study demonstrated limited interobserver agreement in scans taken less than 100 minutes from the known onset of symptoms.Reference Naylor, Churilov, Rane, Chen, Campbell and Yan42 Likewise, two recent ASPECTS interrater agreement studies utilizing both CT and MRI focused on EVT candidates also found insufficient agreement between clinicians.Reference Farzin, Fahed and Guilbert43,Reference Fahed, Lecler and Sabben44 Another modality used to determine the eligibility of patients for EVT, especially in the extended time period (> 6 hours), is perfusion scanning. Unlike, ASPECTS scoring which determines the core infarct based on radiologic changes in the brain parenchyma, perfusion scanning relies on quantifying blood flow to determine the extent of infarcted tissue. A study comparing the two modalities actually found that automated perfusion scanning overestimated the core size when compared to 24 hours post-EVT ASPECTS scans.Reference Tsang, Lenck and Hilditch45
The DEFUSE 3 and EXTEND study established the role of automated perfusion imaging (Rapid processing of PerfusIon and Diffusion [RAPID] software) for selection of patients eligible for medical and mechanical reperfusion.Reference Ma, Campbell and Parsons46,Reference Albers, Marks and Kemp47 RAPID guides neurointerventionalists by effectively mapping out and ischemic core and salvageable penumbra. Because the software provides exact volume estimates for both core and penumbra, there is much less variability in interpretation. However, the added cost of the software for automated perfusion may pose a significant hurdle to public hospitals in developing nations eager to expand their endovascular program. Despite the limitations of ASPECTS, expanding the eligibility of large vessel occlusion ischemic strokes for EVT in terms of the scoring system may be a worthwhile endeavor, especially for these settings. In addition, certain peculiarities with perfusion imaging such as overestimation of penumbral size in patients with chronic ipsilateral carotid stenosis is not an issue for ASPECTS scoring because the latter interprets based on the morphology of the actual brain tissue. More recently, the prevalence of significant clinical-core mismatch and large core (>70 cc) based on RAPID automated perfusion in patients with ASPECTS ≥ 6 was found to be similar in both the early (<6 hours) and late (6–24 hours).Reference Desai, Tonetti and Molyneaux48 This new finding may expand the role of ASPECTS into the late window, especially in places with no access to perfusion imaging.
Limitations
The study has several limitations, the first being the marked heterogeneity found in the EVT group. Second, two of the four studies with data for median ASPECTS show a lower ASPECTS in the best medical therapy group compared to the EVT group (3 vs 5). This may account for the large difference in 90-day outcomes between the two groups as lower ASPECTS are predictive of worse clinical outcomes.Reference Hill49 Third, bias inherent to the retrospective nature of all the studies included could not be avoided. Fourth, the use of both MRI and CT in the determination of initial ASPECTS in the studies reviewed limits the applicability of the study. Disagreement between the two modalities for ASPECTS may be as high as 20%.Reference Hui, Obuchowski and John50 Fifth, there were significantly more diabetic patients in the best medical therapy group. This may have contributed to their poorer outcomes. Sixth, the definition used for sICHReference Yaghi, Willey and Cucchiara26 was not uniform across all the studies included. Seventh, the mean onset to reperfusion time in the EVT group was less than 6 hours. Thus, the results have limited applicability to patients coming in beyond this time. Lastly, misclassification bias may result in some “low ASPECTS” patients actually having a “true” ASPECTS > 6. Similarly, the converse will also have significant implications in the interpretation of our systematic review’s findings. A comparison between the ASPECTS findings of a core laboratory and study investigators for an EVT trial found significant discrepancy between the scores.Reference Fahed, Ben Maacha and Ducroux27 Ongoing randomized clinical trials for EVT on patients with large core infarcts that utilize a core laboratory with uniform parameters will hopefully provide more conclusive data on the subject.Reference Yoshimura28,Reference Bendszus30-32 . Only data from these studies can definitively establish if EVT is really effective in achieving superior outcomes in low ASPECTS patients. These studies will also hopefully shed light on difference in outcomes in the low ASPECTS subgroups, that is, ASPECTS of 0–2 and 3–5.
Conclusions
This meta-analysis of 9 studies including 1,196 patients demonstrates a trend toward higher sICH. Despite this, a significant proportion patients undergoing EVT still achieved good functional outcomes at 90 days. However, the results of the study should be interpreted with caution as all the studies included were observational studies which are inherently biased. Randomized controlled trials are needed to establish the utility of EVT for this subset of patients.
Disclosures
Dr. Bharatha has modest honoraria from Medtronic. Otherwise, the authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Contributors
Study inception: JDBD, AAD, KP, AB. Data extraction: JDBD, GB, KP, AAD. Data analysis: JDBD, KP, AAD. Interpretation: JDBD, AAD, KP, JAH, KC, AK, AB. Initial draft of manuscript: JDBD, KP, AAD. Review and editing: JDBD, AAD, KP, JAH, KC, AK, AB. Supervision: JAH, KC, AK, AB
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.71.







