Theories about the aetiology of schizophrenia continue to develop. It is likely to be multifactorial with genetic and environmental factors interacting or acting alone to increase the individual's risk for developing schizophrenia. At the beginning of the 20th century there was considerable interest in a possible link between ear disease and insanity. Bryant Reference Bryant1 wrote a review citing authors from as early as 1890 who thought that ear disease itself could cause insanity and even secondary dementia. Amberg Reference Amberg2 suggested that the proximity of the ear to the brain was of signal importance and Toubert Reference Toubert3 believed that ‘a suppurating ear is a permanent source of irritation for the brain’. Robinson Reference Robinson4 reported rates of ear disease in 66% of 200 certified insane people.
This hypothesis that an ear infection could cause irritation to the overlying brain has received little interest. Instead, attention has been paid to the role of hearing impairment in the development of paranoid disorders in the elderly. Cooper et al Reference Cooper, Curry, Kay, Garside and Roth5–Reference Cooper7 showed that elderly patients with paranoid disorders had a greater degree of hearing loss and were more often socially deaf than controls with affective illness, who resembled the general population. These studies also showed that chronic middle-ear disease was the most common cause of deafness in elderly patients with paranoid disorders, which may support the above hypotheses of over a century ago.
The temporal lobes, in particular the left temporal lobe, which lies directly above the middle ear, have been implicated in the neuropathology of schizophrenia. Flor-Henry Reference Flor-Henry8 initially reported that the schizophrenia-like psychoses of epilepsy are more common in temporal lobe epilepsy with a left-sided focus. Others have observed increased dopamine in the left amygdala, Reference Reynolds9 decreased left parahippocampal width Reference Brown, Colter, Corsellis, Crow, Frith, Jagoe, Johnstone and Marsh10 and ventricular enlargement limited to the left temporal horn Reference Crow, Ball, Bloom, Brown, Bruton, Colter, Frith, Johnstone, Owens and Roberts11 in people with schizophrenia. It is known that in severe otitis media and mastoiditis spread of infection can occur intracranially through the temporal bone, Reference Ludman and Booth12,Reference Colman, Hall and Colman13 which would support the hypotheses cited by Bryant. Reference Bryant1
A patient described by Mason & Winton Reference Mason and Winton14 developed schizophrenia within 2 months of right mastoid surgery. He was left-handed, left-footed, left-eye dominant and wrote with his left hand non-inverted, Reference Levy and Reid15 suggesting right cerebral dominance. Electroencephalogram revealed runs of abnormally slow activity with focusing in the right temporal region and a skull X-ray demonstrated a communication between the middle ear and middle cranial cavity.
Mason & Winton Reference Mason and Winton14 conducted a case–control study of middle-ear disease in schizophrenia which was based on general practice notes. They found an odds ratio of middle-ear disease in schizophrenia (pre-dating the onset of schizophrenia) of 2.29 (95% CI 1.11–4.71) when compared with non-psychiatric controls.
The aim of the current study was to replicate the case–control study of Mason & Winton Reference Mason and Winton14 using improved methodology to determine the relative risk of middle-ear disease pre-dating the onset of schizophrenia. Additional data were collected to determine presence of deafness and conductive hearing loss in those with schizophrenia and their relationship, if any, to certain symptoms. Our hypothesis was that there would be a higher rate of middle-ear disease pre-dating the onset of schizophrenia than in non-psychiatric controls.
Method
Sample
All patients with a likely diagnosis of schizophrenia in contact with general practitioners in a defined catchment area within West Lancashire were identified (n=99). Power analysis to achieve a power of 80% and a significance level of 5% was based on a prevalence rate of middle-ear disease of 50% Reference Fry and Fry16 and an estimated odds ratio of 2.5. Four controls (total n=396) were needed for each case. They were selected from each case's general practice on the basis of same gender and nearest date of birth. This was to match for gender and age and to limit the effects of seasonality, since an excess of winter births has been proposed as an aetiological factor in schizophrenia. Reference Torrey, Miller, Rawlings and Yolken17 Social class could not easily be controlled for but the effects of this were reduced by selecting the controls from the same general practice as their respective case partners. Middle-ear disease Reference Chadha, Agarwal, Gulati and Garg18 and schizophrenia Reference Harrison, Gunnell, Glazebrook, Page and Kwiecinski19 are more common in lower social classes.
Controls with mental illness were excluded to eliminate other mental illnesses as potential confounding factors and controls with incomplete general practice records were also excluded. For each control excluded an additional control was chosen on the basis of the next nearest date of birth to their respective case partner.
Data collection
History and age at onset of ear pathology was recorded verbatim from the general practice records of all participants by M.R. and G.G. Ear disease was rated as ‘middle-ear disease’ (otitis media, chronic suppurative otitis media and mastoiditis) or ‘other ear disease’ (otitis externa, wax and foreign bodies) by P.M., who was masked to the case/control status of the individual. Ambivalent cases (red drum, otalgia and otorrhoea) were rated as other ear disease.
The consistency of the verbatim accounts recorded by M.R. and G.G. was checked in a reliability study. P.M. rated the presence or absence of middle-ear disease and other ear disease on 74 randomly selected individuals.
The following additional information was collected for participants from their psychiatric case notes and at interview: family history of schizophrenia in a first-degree relative, age and acuteness at onset of schizophrenia, presence or absence of brain damage, and obstetric complications at birth. Symptoms ever experienced were collected from individuals at interview and from their psychiatric case notes using the Syndrome Checklist of the Present State Examination, 9th edition. Reference Wing, Cooper and Sartorius20 An ICD–10 diagnosis of schizophrenia was then made using the Diagnostic Criteria for Research DCR–10. Reference Cooper21
Participants in the case group were questioned about their hearing and memories of ear disease. They were invited to have their hearing examined using Rinne's and Weber's tests and a portable audiometer (after removal of wax). The presence or absence of a hearing deficit was recorded for each person. An estimation of cerebral dominance was achieved using the Edinburgh Handedness Inventory Reference Oldfield22 and hand-writing position. Reference Levy and Reid15
Social class according to the Registrar General's classification of the family of origin of individuals in the case group was also obtained. Information about social class was not available for the controls, as this information is generally not recorded in general practice notes.
Ethical committee approval for the study was obtained from the local ethics committee and written consent was obtained from participants.
Analyses
The data were analysed with STATA version 9 for Windows using conditional logistic regression (clogit) for each set of data. All odds ratios were calculated with 95% confidence intervals. To keep the case–control match in the analyses, whenever a case was excluded, it was excluded together with its respective controls. Owing to lack of data on social class and symptoms for the control group, conditional regression was not possible for some of the analyses and so chi-squared tests and odds ratios were calculated. For the reliability study, the degree of concordance between P.M. and the investigators M.R. and G.G. were examined using Cohen's kappa with 95% confidence intervals.
Results
Of the 99 people in the case group, 57 men and 27 women met ICD–10 diagnostic criteria for schizophrenia. Seven met ICD–10 criteria for delusional disorder and 8 for schizoaffective disorder. The age range for the case group was 18–66 years (mean=42.56, s.d.=12.68) and the age at onset of schizophrenia ranged from 14 to 51 years (mean=29.00, s.d.=9.33).
The reliability study conducted to check the consistency of the data collection from general practice notes had a concordance (Cohen's kappa) of 0.83 (95% CI 0.72–0.95), which represents a high level of interrater agreement.
Table 1 shows the rates of middle-ear disease and other ear disease pre-dating the onset of schizophrenia in the case and control groups. For 35 individuals in the case group the quality of the general practice notes was poor with no notes pre-dating the onset of schizophrenia – these 35 case participants and their respective controls were excluded from the analyses. The rate of middle-ear disease pre-dating the onset of schizophrenia is higher in the case group than in controls (OR=3.68, 95% CI 1.86–7.28). Studying the rates of bilateral, right- and left-sided middle-ear disease pre-dating the onset of schizophrenia in the case and control groups reveals a particularly striking increase in the odds ratio to 4.15 (95% CI 2.08–8.29) for left-sided middle-ear disease.
Table 1 Ear disease pre-dating the onset of schizophrenia
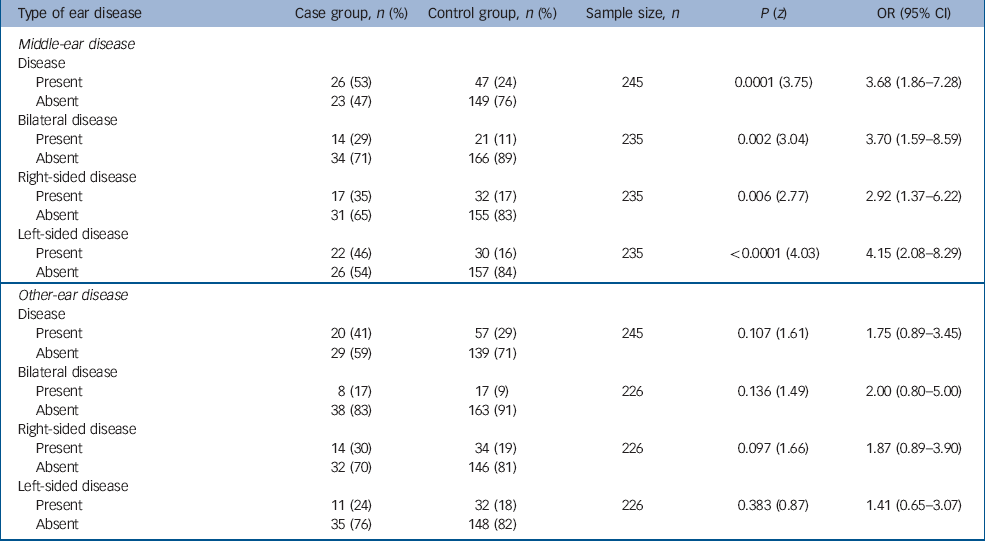
| Type of ear disease | Case group, n (%) | Control group, n (%) | Sample size, n | P (z) | OR (95% CI) |
|---|---|---|---|---|---|
| Middle-ear disease | |||||
| Disease | |||||
| Present | 26 (53) | 47 (24) | 245 | 0.0001 (3.75) | 3.68 (1.86-7.28) |
| Absent | 23 (47) | 149 (76) | |||
| Bilateral disease | |||||
| Present | 14 (29) | 21 (11) | 235 | 0.002 (3.04) | 3.70 (1.59-8.59) |
| Absent | 34 (71) | 166 (89) | |||
| Right-sided disease | |||||
| Present | 17 (35) | 32 (17) | 235 | 0.006 (2.77) | 2.92 (1.37-6.22) |
| Absent | 31 (65) | 155 (83) | |||
| Left-sided disease | |||||
| Present | 22 (46) | 30 (16) | 235 | <0.0001 (4.03) | 4.15 (2.08-8.29) |
| Absent | 26 (54) | 157 (84) | |||
| Other-ear disease | |||||
| Disease | |||||
| Present | 20 (41) | 57 (29) | 245 | 0.107 (1.61) | 1.75 (0.89-3.45) |
| Absent | 29 (59) | 139 (71) | |||
| Bilateral disease | |||||
| Present | 8 (17) | 17 (9) | 226 | 0.136 (1.49) | 2.00 (0.80-5.00) |
| Absent | 38 (83) | 163 (91) | |||
| Right-sided disease | |||||
| Present | 14 (30) | 34 (19) | 226 | 0.097 (1.66) | 1.87 (0.89-3.90) |
| Absent | 32 (70) | 146 (81) | |||
| Left-sided disease | |||||
| Present | 11 (24) | 32 (18) | 226 | 0.383 (0.87) | 1.41 (0.65-3.07) |
| Absent | 35 (76) | 148 (82) |
There appears to be an excess of other ear disease in the case group when compared with controls, but this fails to reach statistical significance. There is no such excess evident with respect to the laterality of other ear disease.
Owing to the lack of additional data on controls it was not possible to perform regression analyses to study the relative effects of other potential aetiological factors. However, on repeating the analyses with the exclusion of individuals in the case group (and their respective controls) with other potential aetiological factors such as family history, obstetric complications and brain damage, little difference is seen from the results presented in Table 1.
The age distribution for the first episode of middle-ear disease pre-dating the onset of schizophrenia in the case group was 1–48 years (mean=15.38, s.d.=12.22). In the controls, the range was 3 months to 39 years (mean=9.99 years, s.d.=9.40). The time interval between this episode of middle-ear disease and the onset of schizophrenia was 1–41 years (mean=16.34, s.d.=11.20) for those in the case group, and 0–42 years (mean=17.42, s.d.=9.99) for controls.
Of those in the case group, 66 (78.6%) did not appear to have any hearing deficits, 13 (15.5%) had a mild degree of hearing loss and 5 (6.0%) had a marked level of hearing loss. Eighty-one participants in the case group consented to an audiogram. This revealed the presence of conductive deafness in 15 (18.5%), sensorineural deafness in 11 (13.6%) and mixed conductive sensorineural deafness in 3 (3.7%) individuals. Concordance between general practice notes (middle-ear disease) and presence of conductive deafness was low (Cohen's kappa=0.13, 95% CI 0.08–0.35) mainly because most incidences of middle-ear disease did not lead to conductive deafness.
Symptoms collected for the case group using the Syndrome Checklist were analysed to see whether any symptoms correlated with middle-ear disease pre-dating onset of schizophrenia or hearing loss. No statistically significant correlations were found for any of the symptoms with the exception of auditory hallucinations. Table 2 shows the rates of middle-ear disease and rates of hearing loss and the presence of auditory hallucinations. Auditory hallucinations are significantly associated with middle-ear disease pre-dating schizophrenia (OR=6.40, 95% CI 1.19–34.29) and this is particularly striking for middle-ear disease on the side of cerebral dominance (OR=10.00, 95% CI 1.15–86.88). Hearing loss does not appear to be associated with auditory hallucinations.
Table 2 Case participants with auditory hallucinations and ear disease pre-dating onset of schizophrenia

| Type of ear disease | Auditory hallucinations, n (%) | No auditory hallucinations, n (%) | Sample size, n | P (χ2) | OR (95% CI) |
|---|---|---|---|---|---|
| Whole sample | |||||
| Hearing loss | |||||
| Present | 13 (21) | 5 (22.7) | 84 | 0.86 (0.03) | 0.90 (0.28-26.44) |
| Absent | 49 (79) | 17 (77.3) | |||
| Complete notes only | |||||
| Hearing loss | |||||
| Present | 6 (15.4) | 2 (20) | 49 | 0.72 (0.124) | 0.73 (0.12-4.30) |
| Absent | 33 (84.6) | 8 (80) | |||
| Middle-ear disease | |||||
| Present | 24 (61.5) | 2 (20) | 49 | 0.019 (5.51) | 6.40 (1.19-34.29) |
| Absent | 15 (38.5) | 8 (80) | |||
| Bilateral middle-ear disease | |||||
| Present | 13 (34.2) | 1 (10) | 48 | 0.13 (2.25) | 4.68 (0.53-41.07) |
| Absent | 25 (65.8) | 9 (90) | |||
| Left-sided middle-ear disease | |||||
| Present | 20 (52.6) | 2 (20) | 48 | 0.065 (3.40) | 4.44 (0.83-23.73) |
| Absent | 18 (47.4) | 8 (80) | |||
| Right-sided middle-ear disease | |||||
| Present | 16 (42.1) | 1 (10) | 48 | 0.059 (3.57) | 6.55 (0.75-57.00) |
| Absent | 22 (57.9) | 9 (90) | |||
| Dominant side middle-ear disease | |||||
| Present | 20 (52.6) | 1 (10) | 48 | 0.016 (5.85) | 10.00 (1.15-86.88) |
| Absent | 18 (47.4) | 9 (90) |
Table 3 shows the social class distribution of those in the case group with and without ear disease pre-dating the onset of schizophrenia. The case group included no one belonging to social classes I (professional), II (managerial and technical) and VI (armed forces). There appears to be an excess of people with middle-ear disease in the lower social classes than those without middle-ear disease. When the data are dichotomised into higher and lower classes (classes IIIN–IV and class V, Table 3), this excess fails to reach statistical significance. This is not evident for left-sided middle-ear disease or for other ear disease.
Table 3 Case participants' social class and ear disease pre-dating the onset of schizophrenia
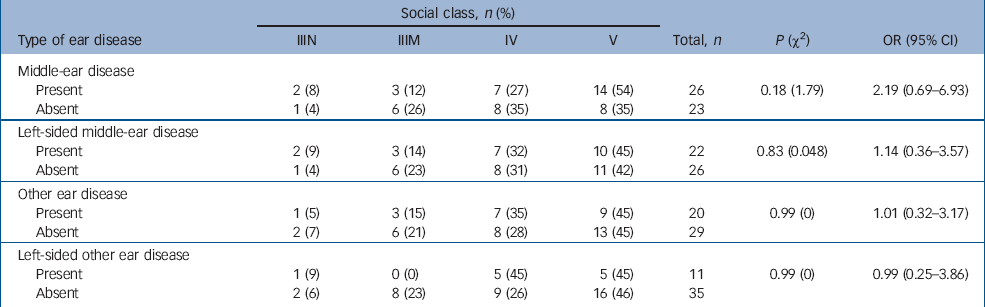
| Social class, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Type of ear disease | IIIN | IIIM | IV | V | Total, n | P (χ2) | OR (95% CI) |
| Middle-ear disease | |||||||
| Present | 2 (8) | 3 (12) | 7 (27) | 14 (54) | 26 | 0.18 (1.79) | 2.19 (0.69-6.93) |
| Absent | 1 (4) | 6 (26) | 8 (35) | 8 (35) | 23 | ||
| Left-sided middle-ear disease | |||||||
| Present | 2 (9) | 3 (14) | 7 (32) | 10 (45) | 22 | 0.83 (0.048) | 1.14 (0.36-3.57) |
| Absent | 1 (4) | 6 (23) | 8 (31) | 11 (42) | 26 | ||
| Other ear disease | |||||||
| Present | 1 (5) | 3 (15) | 7 (35) | 9 (45) | 20 | 0.99 (0) | 1.01 (0.32-3.17) |
| Absent | 2 (7) | 6 (21) | 8 (28) | 13 (45) | 29 | ||
| Left-sided other ear disease | |||||||
| Present | 1 (9) | 0 (0) | 5 (45) | 5 (45) | 11 | 0.99 (0) | 0.99 (0.25-3.86) |
| Absent | 2 (6) | 8 (23) | 9 (26) | 16 (46) | 35 | ||
Discussion
The results of Mason & Winton, Reference Mason and Winton14 who found an odds ratio for middle-ear disease pre-dating the onset of schizophrenia of 2.29 (95% CI 1.11–4.71) when compared with controls, have been replicated in this study (OR=3.68, 95% CI 1.86–7.28). The absence of a statistically significant excess of other ear disease pre-dating the onset of schizophrenia suggests that nosocomial factors do not account for this excess of middle-ear disease. However, these analyses do not have adequate statistical power to be confident of a negative finding and there appears to be more other ear disease in those with schizophrenia than in controls.
Social class, which was not a confounding factor in the Mason & Winton study, Reference Mason and Winton14 may contribute to the higher odds ratio of middle-ear disease pre-dating schizophrenia in this study, as we found a relative excess of middle-ear disease in individuals in the case group from lower social classes. Both middle-ear disease Reference Chadha, Agarwal, Gulati and Garg18 and schizophrenia Reference Harrison, Gunnell, Glazebrook, Page and Kwiecinski19 are more common in lower social classes. It was difficult to control for the effects of social class in this study because there was no information on social class for the control group. However, the potential effects of social class were minimised by selecting controls from the same general practices as their case partners. The area of West Lancashire in which this study was based is a fairly uniform area of high socio-economic deprivation, which would again minimise the potential confounding factor of social class.
One would expect the rates of other ear disease to be in excess in the lower social classes as diseases such as otitis externa, like otitis media, are associated with lower socio-economic status. Reference Chadha, Agarwal, Gulati and Garg18 This was not found in this study, suggesting that social class may not be such an important confounding factor.
In addition, no excess of left-sided middle-ear disease was found in participants from lower social classes. The striking excess of left-sided middle-ear disease pre-dating the onset of schizophrenia (OR=4.15, 95% CI 2.07–8.29) is unlikely to be accounted for by nosocomial factors or social class, and is of particular interest given the evidence linking the left temporal lobe with schizophrenia. Reference Flor-Henry8–Reference Crow, Ball, Bloom, Brown, Bruton, Colter, Frith, Johnstone, Owens and Roberts11 It is known that in severe otitis media and mastoiditis, spread of infection intracranially can occur through the temporal bone by bone necrosis, congenital dehiscences and fracture lines, as well as through thrombophlebitis or via perivascular sheaths. Reference Ludman and Booth12,Reference Colman, Hall and Colman13 It is not unreasonable to speculate that severe middle-ear disease may make a person vulnerable to developing schizophrenia later in life, or that middle-ear disease and schizophrenia may share some aetiological factors. It is possible that there is evidence to support the authors cited by Bryant Reference Bryant1 who thought that ear disease itself could cause insanity and even secondary dementia. However, the broad distribution of time between the first recorded episode of middle-ear disease and the onset of schizophrenia (1–41 years) makes it difficult to speculate about potential mechanisms.
If middle-ear disease can predispose to schizophrenia by inflammatory damage to the overlying temporal lobe, then one would expect to see fibrillary gliosis in the post-mortem brains of people with schizophrenia. The lack of gliosis found in schizophrenia Reference Crow, Ball, Bloom, Brown, Bruton, Colter, Frith, Johnstone, Owens and Roberts11,Reference Roberts, Colter, Lofthouse, Bogerts, Zech and Crow23–Reference Bruton, Crow, Frith, Johnstone, Owens and Roberts26 has been one of the main arguments in favour of the neurodevelopmental hypothesis of schizophrenia, and it does not support the above hypothesis. However, this absence of gliosis is not a particularly robust finding. Earlier studies Reference Nieto, Escobar and Minckler27–Reference Stevens29 all reported an excess of gliosis in schizophrenia, and the later studies have been criticised for using immunochemical methods for detecting gliosis which may lack sensitivity. Reference Stevens, Casanova and Bigelow30,Reference Stevens, Casanova, Poltorak, Germain and Buchan31
Analysis of the data excluding other potential aetiological factors (family history, obstetric complications and brain damage) did not replicate the results of Mason & Winton, Reference Mason and Winton14 who found increased rates of middle-ear disease when case participants with other potential aetiological factors were excluded. Those findings were not particularly robust, however, given the small sample sizes, and it is more likely that risk factors for schizophrenia operate cumulatively rather than individually in the aetiology of schizophrenia. In the current study, lack of information for other potential aetiological factors in the control group meant that analytical statistical methods such as regression analyses could not be used to study the relative effect size of different aetiological factors.
Deafness is also a potential link between ear disease and schizophrenia. In particular, certain symptoms such as paranoid delusions Reference Cooper, Curry, Kay, Garside and Roth5–Reference Cooper7 and auditory hallucinations Reference Hecaen and Ropert32,Reference Keshavan, David, Steingard and Lishman33 have been linked with hearing impairment, and the use of a hearing aid can reduce psychotic symptoms in patients with deafness. Reference Almeida, Förstl, Howard and David34 The current study does not support these hypotheses, with no statistically significant association found between hearing loss and auditory hallucinations. Although the statistical power of this analysis is low, it is supported in the studies of Howard et al Reference Howard, Almeida and Levy35 and Almeida et al, Reference Almeida, Howard, Levy and David36 who found no association between hearing loss and auditory hallucinations in patients with late paraphrenia. The statistically significant association between middle-ear disease pre-dating the onset of schizophrenia and auditory hallucinations is particularly striking, especially for middle-ear disease on the side of cerebral dominance, adding to the speculation that damage to the overlying temporal lobe may be of aetiological significance.
Methodological issues
The methodology used in the current study has its limitations, in addition to the potential confounding factor of social class. In particular, the quality of general practice notes makes it difficult to operationalise the definitions of middle-ear disease. Ambivalent cases (red drum, otalgia and otorrhoea) were excluded from our definition of middle-ear disease, although in many of these cases middle-ear disease may have been present. The quality of information in the notes made it too difficult to make a rating of severity of middle-ear disease, which would have allowed further statistical analysis. Statistical power is low for a number of the analyses because complete general practice notes were lacking for 35 people in the case group. All the analyses were repeated using the whole sample. These analyses yielded remarkably similar, statistically significant results, albeit with lower odds ratios. This is all the more remarkable given the study design which was biased in favour of the null hypothesis (controls were excluded if they did not have complete notes and alternative controls were found). Low statistical power is a major drawback for the analyses of symptoms, and clearly these analyses should be repeated on larger samples. Bias did not seem to be a factor influencing these results given the high level of interrater agreement for the data collection from general practice notes. In addition, the presence of middle-ear disease/other ear disease was rated masked to the diagnostic status of the participant.
Conclusion
It appears that there is an association between middle-ear disease and schizophrenia. The previous study of Mason & Winton Reference Mason and Winton14 has been replicated with better methodology, and the association between middle-ear disease and schizophrenia is greater. It is particularly striking that it is left-sided middle-ear disease pre-dating the onset of schizophrenia which has the greatest association, given what is known about the left temporal lobe and schizophrenia. Middle-ear disease may be another aetiolgical factor which increases one's vulnerability to developing schizophrenia. This association is worthy of further research.
Acknowledgements
The authors are grateful to the patients participating in this study, their general practitioners, members of the community mental health team who gave their assistance and the Audiology Department at Ormskirk & District General Hospital for the loan of their portable audiometer. They also thank the Evidence-based Practice Centre at St Catherine's Hospital, Birkenhead, for help with literature searches, statistics and advice on earlier manuscripts.






eLetters
No eLetters have been published for this article.