Introduction
Bipolar disorder is a complex and chronic mood disorder characterized by alternating or intertwining episodes of mania, hypomania, and depression.Reference Grande, Berk, Birmaher and Vieta1 Of these mood states, the depressive phase is the most enduring and disabling feature of the disorder as patients with bipolar disorder spend up to half of their illness lifetime with depressive symptoms, approximately three times more than their time with manic/hypomanic symptoms.Reference Miller, Dell’Osso and Ketter2, Reference Kupka, Altshuler and Nolen3 Further, bipolar and unipolar depression is strongly and consistently associated with increased work loss and decreased productivityReference Kessler, Akiskal and Ames4; this association is less defined with mania/hypomania.Reference Simon, Ludman, Unutzer, Operskalski and Bauer5 Treatment of bipolar depression remains a challenge for clinicians, especially given that there are fewer treatment options available for treating bipolar depression compared with mania.Reference Miller, Dell’Osso and Ketter2 In addition, patients with bipolar depression can also suffer from mixed symptomsReference Grande, Berk, Birmaher and Vieta1 that can further complicate treatment.
The criteria for mixed episodes have changed over time. The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV-TR)6 required the contemporaneous presence of a threshold manic and major depressive episode; however, this definition was found to be too restrictive as it did not reflect the range of presentations for bipolar disorder and excluded patients who were experiencing either a major depressive, manic, or hypomanic episode with varying degrees of subsyndromal symptoms of the opposite mood state.7-9 To be more clinically relevant, the definition was broadened in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)10 with the “with mixed features” specifier, defined as patients meeting criteria for either a major depressive, manic, or hypomanic episode with 3 or more symptoms (without meeting the full criteria as previously required by DSM-IV-TR) of the opposite mood state. Using various modified DSM-5 definitions, it has been estimated that up to 40% (depending on source) of patients with bipolar disorder will experience mixed mood symptoms during manic, hypomanic, or depressive episodes.Reference Rosenblat and McIntyre8, Reference Fornaro, Stubbs and De Berardis9, Reference McIntyre, Soczynska and Cha11, Reference Miller, Suppes and Mintz12 The presentation of bipolar depression with mixed features is complex, and there is debate in the literature on whether the symptoms of mixed features are fully captured by the DSM–5 diagnostic criteria.13-15 It should be noted that a number of studies have suggested that the presence of as few as 3 symptoms or less may be sufficient to define a mixed populationReference Miller, Suppes and Mintz12, Reference Goldberg, Perlis and Bowden16; as such, the criteria for mixed features remains an iterative process.
Bipolar depression with mixed symptoms is associated with greater symptom severity, higher rates of mood episode recurrence and comorbidities, worse clinical outcomes, lower rates of treatment response, and increased risk of suicidality.Reference Betzler, Stover, Sterzer and Kohler17 Currently, no agent has been approved by the FDA or EMA for the treatment of patients with bipolar disorder exhibiting a mixed features specifier. In the absence of approved treatments for mixed features, antidepressants are commonly used to treat mixed symptoms, but they are generally ineffective and may raise the risk of manic episodes in this population.Reference Pacchiarotti, Bond and Baldessarini18, Reference Fornaro, Anastasia and Novello19 In addition, as bipolar depression is frequently misdiagnosed as unipolar depressionReference Post20, antidepressants may also be incorrectly prescribed, which can lead to reduced treatment response. There is evidence that atypical antipsychotics, which can lower the severity of both depressive and manic symptoms, may be able to treat bipolar depression with mixed symptoms.Reference Rosenblat and McIntyre8, Reference Fornaro, Stubbs and De Berardis9 It has also been hypothesized that atypical antipsychotics that have affinity for receptors that are thought to modulate depression, such as dopamine D3 and serotonin 5-HT1A, may be effective treatments for bipolar depression.Reference Leggio, Salomone and Bucolo21, Reference Celada, Puig, Amargos-Bosch , Adell and Artigas22 To this end, a panel of experts have recently recommended that atypical antipsychotics should be considered as the initial treatment for patients with mixed depression.Reference Stahl, Morrissette and Faedda23
Cariprazine, a dopamine D3-preferring D3/D2 receptor and 5-HT1A receptor partial agonist, is approved for the treatment of adults with schizophrenia (1.5–6 mg/day; United States and Europe) and manic/mixed episodes associated with bipolar I disorder (3–6 mg/day; United States). Cariprazine has also been recently approved as a monotherapy for bipolar I depression (1.5–3 mg/day; United States) based on efficacy and safety/tolerability results from 3 positive phase II/III randomized, double-blind, placebo-controlled trials in patients with bipolar I depression.24–26 Cariprazine has demonstrated efficacy versus placebo at both poles of the bipolar spectrum and is only the second agent approved to treat episodes of both mania and depression in patients with bipolar I disorder.
The objective of this post hoc analysis was to evaluate the efficacy of cariprazine in patients with bipolar depression, with or without concurrent manic symptoms using pooled data from the 3 aforementioned clinical studies. As these 3 studies excluded patients experiencing moderate-to-severe manic symptoms, we used slightly broader criteria in our post hoc analyses to identify patients with concurrent manic and depressive symptoms (Young Mania Rating Scale [YMRS] total score ≥4) than what is specified by the DSM-5 to define mixed features.
Methods
Post hoc analyses were performed using pooled data from 3 phase II/III randomized, double-blind, placebo-controlled trials in patients with bipolar depression (MD-56 [NCT01396447], MD-53 [NCT02670538], MD-54 [NCT02670551]). All 3 studies were conducted in compliance with the International Conference on Harmonisation Guidances on General Considerations for Clinical Trials and Good Clinical Practice and the Declaration of Helsinki. These studies were also approved by institutional review boards or ethics committees and government agencies. All participants provided written informed consent after receiving a complete description of the studies. The studies were conducted between March 2016 and January 2018 (MD-53), March 2016 and July 2017 (MD-54), and July 2011 and January 2014 (MD-56).
Patients
These multiregional studies, conducted in the United States and 12 other countries, enrolled adult patients 18 to 65 years of age. Patients were eligible to enroll if they had a DSM-IV-TR (MD-56) or DSM-5 (MD-53 and MD-54) diagnosis of bipolar I disorder with a current major depressive episode (duration ≥4 weeks and ≤12 weeks) and met the following inclusion criteria at baseline: 17-item Hamilton Depression Rating Scale (HAMD17)Reference Hamilton27–Reference Miller, Bishop, Norman and Maddever29 total score ≥20; item 1 depressed mood score ≥2; Clinical Global Impressions-Severity (CGI-S)Reference Guy and Guy30 score ≥4; and YMRSReference Young, Biggs, Ziegler and Meyer31 total score ≤10 (MD-56) or ≤12 (MD-53 and MD-54). For studies MD-53 and MD-54, patients were also required to be currently treated as an outpatient at the time of enrollment. Other exclusion criteria included if the patient were at risk for suicide (recent suicide attempt or as judged by the investigator based on psychiatric interview or the Columbia–Suicide Severity Rating Scale [C-SSRS]Reference Posner, Brown and Stanley32), or if the patient had a history of substance dependence within 6 months prior to the study.
Study design
All 3 studies required patients to undergo screening and washout for up to 14 days; eligible patients were randomly assigned to receive 6 (MD-53 and MD-54) or 8 weeks (MD-56) of double-blind treatment with placebo or fixed doses of cariprazine (0.75 [in MD-56 only], 1.5, or 3 mg/day). In study MD-56, all patients randomized to the cariprazine treatment groups were initiated on a dose of 0.5 mg/day and the dosage was increased to 0.75 mg/day on day 3; in the 1.5- and 3-mg/day dose groups, the dosage was increased to 1 mg/day on day 5 and then to 1.5 mg/day on day 8, and in the 3-mg/day dose group, the dosage was further increased to 3 mg/day on day 15. In studies MD-53 and MD-54, all patients randomized to receive cariprazine were initiated on a dose of 1.5 mg/day; in the 3-mg/day dose group, the dosage was increased to 3 mg/day on day 15. The primary efficacy parameter in all 3 studies was change from baseline in the Montgomery-Åsberg Depression Rating Scale (MADRS)Reference Montgomery and Asberg33 total score at week 6. The secondary efficacy parameter in all 3 studies was change from baseline in the CGI-S score at week 6. YMRS total score was also assessed and was included to determine if there was any worsening of manic symptoms during the studies.
Definition of concurrent manic symptoms
Post hoc analyses were based on the subgroups of patients with concurrent manic symptoms or without concurrent manic symptoms. Patients were determined to have concurrent manic symptoms if their baseline YMRS total score was ≥4; patients were categorized as not having concurrent manic symptoms if they had a baseline YMRS total score <4. Previous studies have suggested that a baseline YMRS total score ≥4 defines a patient population (patients with concurrent manic symptoms) with distinct response characteristics versus patients with baseline YMRS total score <4.34–36
Post hoc analyses
Efficacy versus placebo was assessed for the pooled cariprazine 1.5 and 3 mg/day fixed-dose groups. Analyses included least squares (LS) mean change from baseline to week 6 in MADRS total and individual item score, HAMD17 total score, CGI-S score, and YMRS total score; these efficacy parameters were all analyzed using a mixed-effects model for repeated measures (MMRM). Rates of MADRS response (≥50% improvement) and remission (total score ≤10), HAMD17 (total score ≤7) remission, and CGI-S remission (total score ≤2) were analyzed using logistic regression with last observation carried forward (LOCF) to impute missing values. Statistical significance was set at a level of .05. Treatment-emergent mania was defined as having YMRS total score ≥16 at any postbaseline visit.
RESULTS
Baseline characteristics and patient disposition
A total of 1383 patients with bipolar depression were included in the ITT population; 808 patients (58.4%) were identified to have concurrent manic symptoms, and 575 patients (41.6%) did not have concurrent manic symptoms. Baseline characteristics were generally similar between patient and treatment groups except for YMRS total score, which was higher in patients with concurrent manic symptoms versus patients without concurrent manic symptoms (Table 1). The duration of current depressive episode, number of depressive and manic/mixed episodes during lifetime, and number of mood episodes (including manic, mixed, hypomanic, depressive) during the past year were also similar between patient and treatment groups (Supplemental Table 1). At baseline, individual YMRS items with the highest mean scores were the sleep and irritability items; mean baseline scores for other YMRS items were low (Figure 1).
Table 1. Baseline characteristics.
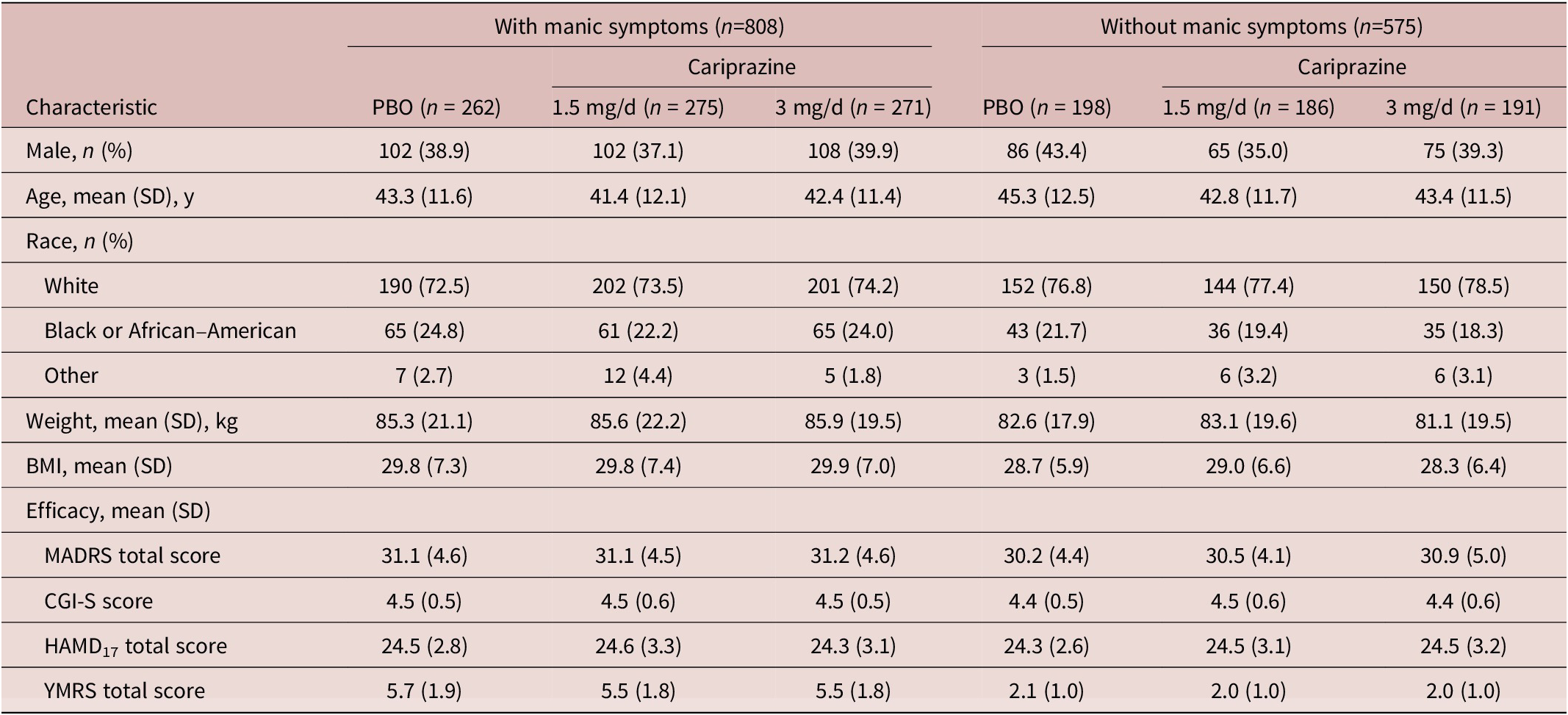
Abbreviations: BMI, body mass index; CGI-S, Clinical Global Impressions-Severity Rating Scale; HAMD17, 17-item Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; PBO, placebo; SD, standard deviation; SE, standard error; YMRS, Young Mania Rating Scale.
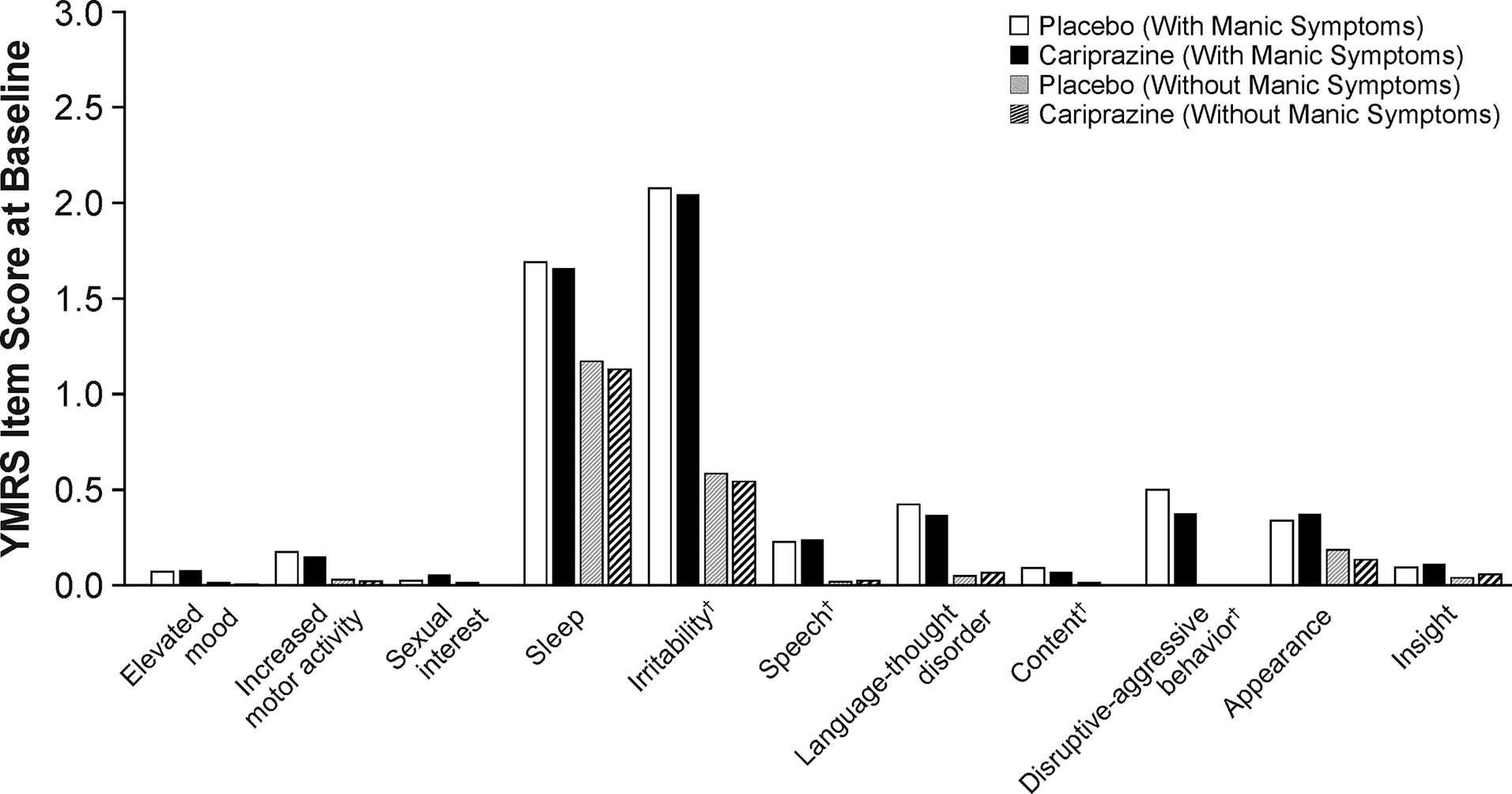
Figure 1. Baseline YMRS individual item scores. †Core items scored with range of 0–8; all other items scored with a range of 0–4.
Efficacy on depressive symptoms
The cariprazine treatment groups showed significantly greater improvement than the placebo group in MADRS total score beginning within the first 2 weeks and persisting to week 6 in patients with ( Figure 2) and patients without ( Figure 2) concurrent manic symptoms. In patients with concurrent manic symptoms, significant improvement over placebo was seen in the cariprazine 3 mg/day dose group starting at week 1 (p < .05). At week 6, the LS mean difference (LSMD) versus placebo was statistically significant in favor of cariprazine for all cariprazine dose groups in patients with concurrent manic symptoms and for the cariprazine 1.5 mg/day dose group in patients without concurrent manic symptoms ( Table 2). On the MADRS total score change at at week 6, differences between cariprazine doses were not statistically significant in patients with (LSMD = 0.3476; p = .6830) and without concurrent manic symptoms (LSMD = −0.1449; p = .1413).
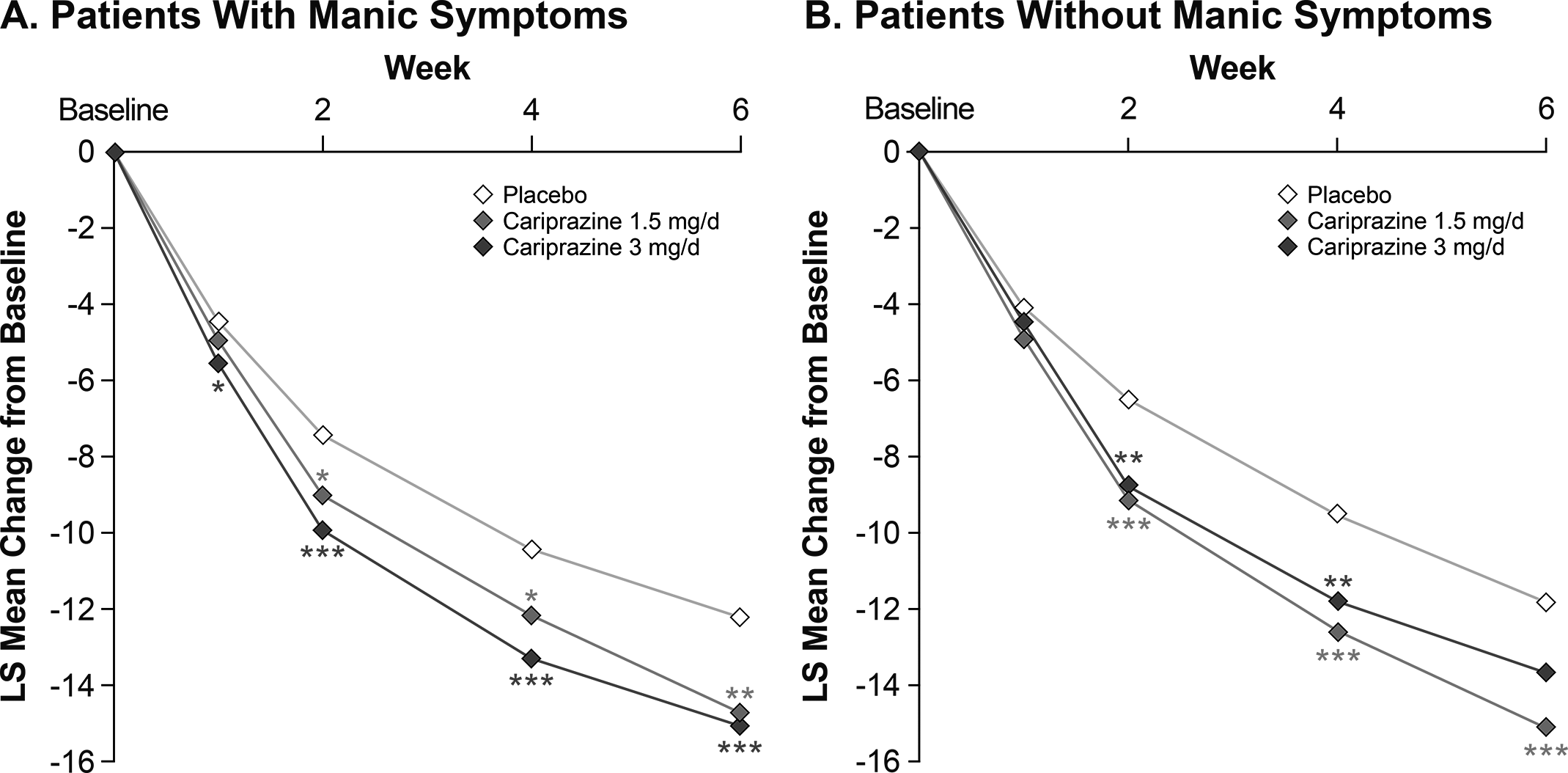
Figure 2. By-week change in MADRS total score from baseline to week 6 in patients (A) with or (B) without manic symptoms (MMRM). *p < .05, **p < .01, ***p ≤ .001 vs placebo. LS, least squares; MADRS, Montgomery-Åsberg Depression Rating Scale; MMRM, mixed-effects model for repeated measures.
Table 2. Summary of Outcomes.
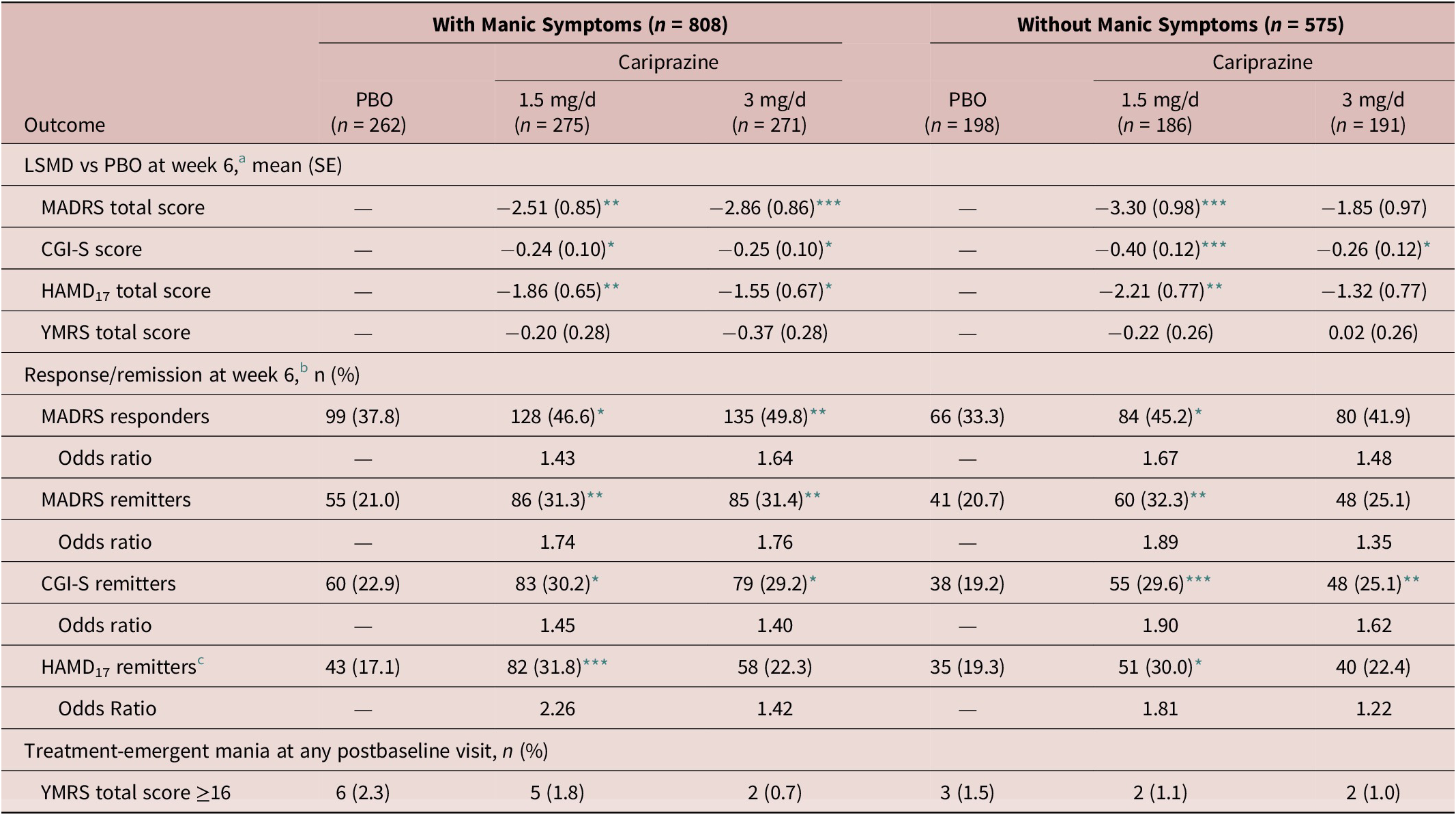
a Analyzed using MMRM.
b Logistic regression using LOCF.
c Number of patients included in the HAMD17 remission analysis were different than the ITT population (patients with manic symptoms: PBO=251, 1.5 mg/day = 258, 3 mg/day = 254; patients without manic symptoms: PBO=181, 1.5 mg/day = 170, 3 mg/day = 179).
Note: *p < .05, **p < .01, ***p ≤ .001 vs placebo.
Abbreviations: CGI-S, Clinical Global Impressions-Severity Rating Scale; HAMD17, 17-item Hamilton Depression Rating Scale; ITT, intent-to-treat; LOCF, last observation carried forward; LSMD, least squares mean difference; MADRS, Montgomery-Åsberg Depression Rating Scale; MMRM, mixed model for repeated measures; PBO, placebo; SE, standard error; YMRS, Young Mania Rating Scale.
Cariprazine demonstrated significantly greater improvement than placebo across a range of MADRS individual items in patients with ( Figure 3) and without ( Figure 3) concurrent manic symptoms. There were slight differences in the number of significant items in each subgroup based on the dose of cariprazine. In patients with manic symptoms, both doses of cariprazine demonstrated efficacy versus placebo on the items of apparent sadness, reported sadness, reduced appetite, concentration difficulties, and lassitude; cariprazine 1.5 mg/day also demonstrated efficacy versus placebo on the inner tension item. In patients without concurrent manic symptoms, cariprazine 1.5 mg/day demonstrated efficacy versus placebo on 8 items (apparent sadness, reported sadness, reduced sleep, reduced appetite, concentration, lassitude, inability to feel, and pessimistic thoughts); cariprazine 3 mg/day demonstrated efficacy versus placebo on the apparent sadness and inability to feel items.
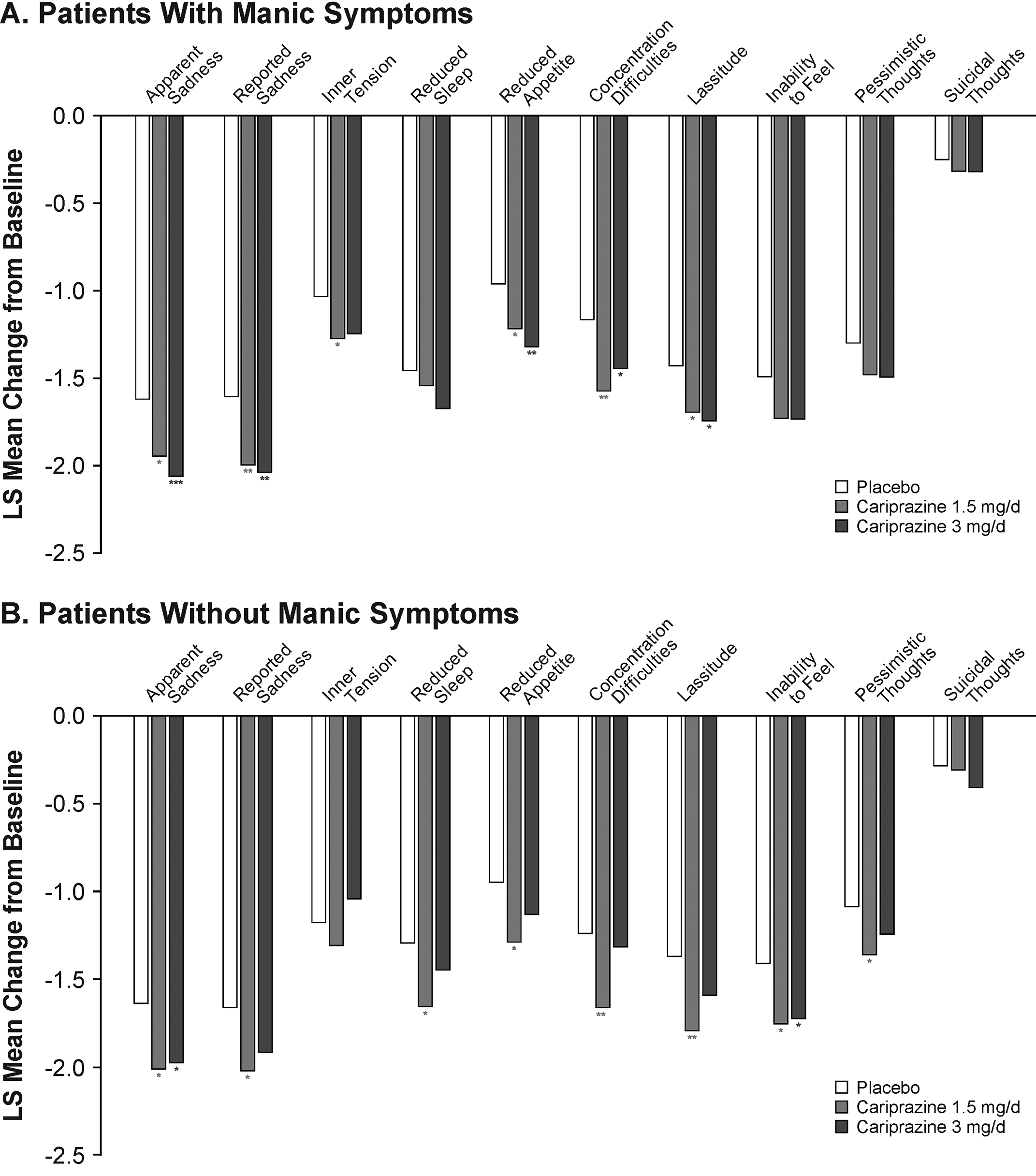
Figure 3. Change in MADRS individual item score from baseline to week 6 in patients (a) with or (b) without manic symptoms (MMRM). *p < .05, **p < .01, ***p < .001 vs placebo. LS, least squares; MADRS, Montgomery-Åsberg Depression Rating Scale; MMRM, mixed-effects model for repeated measures.
In patients with concurrent manic symptoms, the percentage of patients who met MADRS response and remission criteria at week 6 was statistically higher in all cariprazine dose groups (response: 1.5 mg/day, number needed to treat [NNT] = 12; 3 mg/day, NNT = 9; remission: 1.5 mg/day, NNT = 10; 3 mg/day, NNT = 10; all, p < .05) versus placebo ( Table 2). In patients without concurrent manic symptoms, the percentage of patients who met MADRS response and remission criteria at week 6 was statistically higher in the cariprazine 1.5 mg/day group (response: NNT = 9; remission: NNT = 9; both, p < .05) versus placebo ( Table 2). The rate of MADRS response and remission at week 6 was numerically greater than placebo in the cariprazine 3 mg/day group, but the differences were not statistically significant (response: p = .0655; remission: p = .2207).
The cariprazine treatment groups showed significantly greater improvement than the placebo group in HAMD17 total score beginning after 2 weeks of treatment and persisting to week 6 in patients with and without concurrent manic symptoms (Supplemental Figure 1, Table 2). The percentage of patients who met HAMD17 remission criteria at week 6 was significantly higher in favor of cariprazine versus placebo for patients with concurrent manic symptoms in the cariprazine 1.5 mg/day dose group (NNT = 7; P < .01) and for patients without concurrent manic symptoms in the 1.5 mg/day dose group (NNT = 10; p < .05). The HAMD17 remission rate in patients treated with cariprazine 3 mg/day were numerically greater than placebo, but the differences were not significant ( Table 2). On the HAMD17 total score change at week 6, differences between cariprazine doses were not statistically significant in patients with (LSMD = ‑0.3108; p = .6350) and without concurrent manic symptoms (LSMD = ‑0.8850; p = .2537).
Other efficacy parameters
All cariprazine treatment groups showed significantly greater improvement than the placebo group in the CGI-S score beginning within the first 2 weeks and persisting to week 6 in patients with and without concurrent manic symptoms (Supplemental Figure 2, Table 2). In patients with concurrent manic symptoms, significant improvement over placebo was seen in the 3 mg/day dose group starting at week 1 (p < .05). The percentage of patients who met CGI-S remission criteria at week 6 was statistically significantly higher in all cariprazine dose groups versus placebo ( Table 2). On the CGI-S score change at week 6, differences between cariprazine doses were not statistically significant in patients with (LSMD = 0.0027; p = .9789) and without concurrent manic symptoms (LSMD = ‑0.1445; p = .2312) on the CGI-S score change at week 6.
Rates of treatment-emergent mania were low (<3%) in all treatment groups and numerically lower in the cariprazine groups compared with the placebo group (Table 2). YMRS total scores decreased in all treatment groups for patients with concurrent manic symptoms (placebo = −1.36; 1.5 mg/day = −1.56; 3 mg/day = −1.73) and for patients without concurrent manic symptoms (placebo = ‒0.13; 1.5 mg/day = −0.35; 3 mg/day = −0.11); differences between groups were not statistically significant ( Table 2). On the YMRS total score change at week 6, differences between cariprazine doses were also not statistically significant in patients with (LSMD = 0.1690; p = .5395) and without concurrent manic symptoms (LSMD = −0.2372; p = .3601).
Discussion
Mixed symptoms in bipolar depression are associated with greater symptom severity, higher rates of mood episode recurrence and comorbidities, worse clinical outcomes, lower rates of treatment response, and increased risk of suicidality.Reference Betzler, Stover, Sterzer and Kohler17 Currently, the treatment of bipolar depression with concurrent manic symptoms remains a challenge, and while antidepressants are commonly used, they are generally ineffective and may increase the risk of mood destabilization and manic episodesReference Pacchiarotti, Bond and Baldessarini18, Reference Fornaro, Anastasia and Novello19, Reference Frye, Helleman and McElroy34; thus, having new effective treatment options for this patient population is imperative. On the basis of available evidence and in the absence of approved agents, a panel of experts has recently recommended that atypical antipsychotics should be considered as the initial treatment for patients with mixed depression, Reference Stahl, Morrissette and Faedda23 though some controversary exists regarding the diagnostic criteria.13–Reference Koukopoulos and Sani15 Several reports have identified that the presence of 1–3 definite manic symptoms is clinically relevant.Reference Miller, Suppes and Mintz12, Reference Goldberg, Perlis and Bowden16 Using this broader definition of “with mixed features” in patients with bipolar depression, several studies have suggested that atypical antipsychotics may be an effective treatment in this patient populationReference Tohen, Kanba, McIntyre, Fujikoshi and Katagiri35–37; however, it should be noted that the majority of these studies have been performed post hoc.Reference Rosenblat and McIntyre8, Reference Fornaro, Stubbs and De Berardis9 Our analysis was designed in a similar manner; to be identified as having concurrent manic symptoms, patients had baseline YMRS total score ≥4 but without requiring any minimum number of manic symptoms.
In this post hoc analysis of 3 pooled phase II/III randomized, double-blind, placebo-controlled trials in patients with bipolar depression, more than half of the patients (58.4%) met the criterion for concurrent manic symptoms (YMRS total score ≥4 at baseline); this is relatively consistent with previously reported prevalence rates for mixed symptoms, which can range from 11% to 70% depending on the study and criteria used.Reference Rosenblat and McIntyre8, Reference Fornaro, Stubbs and De Berardis9, Reference McIntyre, Soczynska and Cha11, Reference Miller, Suppes and Mintz12, Reference Goldberg, Perlis and Bowden16 In patients with concurrent manic symptoms, sleep and irritability items had the highest mean baseline scores; this is consistent with previous reports as sleep and irritability have been identified to be common manic symptoms occurring in patients with bipolar depression.Reference Miller, Suppes and Mintz12, Reference Goldberg, Perlis and Bowden16, Reference Judd, Schettler and Akiskal38 Previous reports have also identified that patients with bipolar depression and mixed symptoms are more likely to be female compared with patients without manic symptomsReference Miller, Suppes and Mintz12, Reference Goldberg, Perlis and Bowden16, Reference McIntyre, Cucchiaro, Pikalov, Kroger and Loebel36; however, in our analyses, the sex distributions were similar between patient subgroups.
In patients with bipolar I depression, cariprazine demonstrated efficacy versus placebo in improving depressive symptoms in patient subgroups with and without concurrent manic symptoms as measured by MADRS and HAMD17 total scores. When both patient subgroups are considered, treatment effects were seen on a wide range of depressive symptoms as cariprazine was more effective than placebo on 9 of 10 MADRS individual items. The CGI-S scale, although it is not a direct measure of depressive symptoms, provides a clinician-rated view of a patient’s global functioning and takes into consideration factors such as patient history, symptoms, behavior, and psychosocial condition; overall, the CGI scale is meant to provide a useful outcome to help clinicians determine the clinical relevance of patient change. In these post hoc analyses, cariprazine significantly improved the CGI-S score versus placebo in patients with and without manic symptoms, indicating that cariprazine provided meaningful clinical benefits in patients with bipolar I depression.
On depressive symptoms, both doses of cariprazine (1.5 and 3 mg/day) were consistently more effective than placebo in patients with bipolar depression and concurrent manic symptoms, while only the 1.5 mg dose was consistently more effective than placebo in patients without manic symptoms. The reason for these differential dose effects is unknown; however, one potential explanation could be related to the pharmacology of cariprazine. In mixed presentations, affected individuals exhibit multidimensional psychopathology including cognitive dysfunction, anhedonia, and significant difficulties in affect regulation (eg, anxiety, irritability); drugs with different receptor profiles across their dosing range may have implications for treating patients with such complex symptom profiles.Reference McIntyre39 Pharmacodynamic studies have suggested that the receptor binding profiles for cariprazine do indeed differ depending on dose. For example, in patients with schizophrenia, cariprazine exhibited a greater preference for occupying dopamine D3 versus D2 receptors at lower doses relative to higher doses.Reference Girgis, Slifstein and D’Souza40 Since D3 receptors are preferentially expressed in regions of the brain that are believed to be involved in modulating mood and cognitionReference Leggio, Salomone and Bucolo21, Reference Stahl41, Reference Nakajima, Gerretsen and Takeuchi42, the preferential occupancy of D3 receptors at lower doses may contribute to the efficacy of cariprazine on depressive symptoms in bipolar depression, a population where the effective doses of cariprazine are lower than those explored in the bipolar mania studies. At higher doses, D2 and D3 receptor occupancy is more balanced, Reference Girgis, Slifstein and D’Souza40 which may be beneficial in patients with bipolar mania or mixed features. Unlike the depressive symptom scales, there were no dose effects seen in the CGI-S results as cariprazine demonstrated efficacy versus placebo on the CGI-S in all patient and dose groups, indicating an overall and broad treatment benefit by cariprazine in bipolar depression. Taken together, these results suggest that both doses of cariprazine are effective in improving symptoms in patients with bipolar depression, though clinicians should be aware that different subsets of patients may respond differently to higher versus lower doses of cariprazine.
Patients with mixed features have a higher propensity for relapse, recurrence, and nonrecovery, suggesting that an effective treatment in patients with mixed features may also offer a higher probability of sustaining acute benefits. Some treatments for bipolar depression can exacerbate mania symptoms and the presence of mixed features in bipolar depression has been identified as a risk factor, Reference Niitsu, Fabbri and Serretti43 further highlighting the need for treatments that do not destabilize mood or induce a manic switch in patients with bipolar depression and mixed features. In this study, rates of treatment-emergent mania and YMRS total score change were comparable between the cariprazine and placebo groups in patients with and without concurrent manic symptoms, indicating that cariprazine does not have an increased risk of inducing manic episodes or worsening manic symptoms while improving depressive symptoms in patients with bipolar depression.
Results from this study need to be interpreted within its limitations. For example, this analysis was not a prospective study but rather a post hoc study of previously conducted clinical trials in patients with bipolar I depression. As such, the inclusion and exclusion criteria of the constituent studies may prevent results from being generalizable to certain patient populations. For example, patients with suicidal behavior and comorbid disorders were excluded. In addition, patients with YMRS total score >10 (MD-53 and MD-54) or >12 (MD-56) were excluded from these studies, which limited the severity of concurrent manic symptoms in this population. As a result, this post hoc study was not meant to be and is not an evaluation of the DSM-5 mixed features criteria, but instead a broader criteria of baseline YMRS total score ≥4 to identify patients with concurrent manic symptoms was used. This definition has been previously used to categorize patients as having mixed features, Reference McIntyre, Cucchiaro, Pikalov, Kroger and Loebel36 though the clinical relevance of this definition and similar “mixed features” criteria have not been thoroughly investigated or validated prospectively. Additionally, the YMRS total score analyses should also be interpreted cautiously as the YMRS exclusion criteria may limit the ability to assess and detect meaningful changes in YMRS total score.
Conclusions
In this post hoc study of 3 randomized, double-blind, placebo-controlled trials in patients with bipolar I depression, cariprazine demonstrated efficacy versus placebo in improving depressive symptoms in patient subgroups with and without concurrent manic symptoms. These results suggest that cariprazine may be an appropriate treatment option for patients with bipolar I depression and concurrent manic symptoms, with higher doses potentially more effective in patients with manic symptoms.
Acknowledgements.
Writing and editorial assistance was provided to the authors by Katharine Fang, PhD, of Prescott Medical Communications Group (Chicago, IL), a contractor of Allergan. Statistical analysis support was provided by Yan Zhong, PhD, of Allergan (Madison, NJ).
Funding.
This study was sponsored by Allergan.
Disclosures.
R. McIntyre has received research grants from private industries or non-profit funds, such as the Canadian Institutes of Health Research, China National Natural Research Foundation, Lundbeck, Shire, and Stanley Medical Research Institute; during the last two years, R. McIntyre has also received speaker/consultation fees from Allergan, Janssen-Ortho, Lundbeck, Neuralstem, Neurocrine, Otsuka, Pfizer, Purdue, Shire, Sunovion, and Takeda. T. Suppes has reported in the last 36 months grants from Merck, National Institute of Health, National Institute of Mental Health, National Institute on Drug Abuse, Palo Alto Health Sciences, Pathways Genomics, Stanley Medical Research Institute, VA Cooperative Studies Program, and VA OR&D PRIME Care; consulting fees from Allergan, Inc. and Sunovion Pharmaceuticals Inc.; honoraria from CMEology, Global Medical Education, and Medscape Education; royalties from Jones and Bartlett, Hogrefe Publishing and Wolters Kluwer Health (UpToDate); and travel reimbursement from CMEology, Global Medication Education, and Sunovion Pharmaceuticals, Inc. W. Earley is an employee of Allergan, and shareholder of Allergan, AstraZeneca, and Eli Lilly. M. Patel is an employee of Allergan. Stephen M. Stahl, MD, PhD, is an Adjunct Professor of Psychiatry at the University of California San Diego, Honorary Visiting Senior Fellow at the University of Cambridge, UK and Director of Psychopharmacology for California Department of State Hospitals. Over the past 36 months (January 2016–March 2019), Dr. Stahl has served as a consultant to Acadia, Adamas, Alkermes, Allergan, Arbor Pharmaceuticals, AstraZeneca, Avanir, Axovant, Axsome, Biogen, Biomarin, Biopharma, Celgene, Concert, ClearView, DepoMed, Dey, EnVivo, Ferring, Forest, Forum, Genomind, Innovative Science Solutions, Intra-Cellular Therapies, Janssen, Jazz, Lilly, Lundbeck, Merck, Neos, Novartis, Noveida, Orexigen, Otsuka, PamLabs, Perrigo, Pfizer, Pierre Fabre, Reviva, Servier, Shire, Sprout, Sunovion, Taisho, Takeda, Taliaz, Teva, Tonix, Trius, Vanda and Vifopharma; He has been a board member to RCT Logic and Genomind; he has served on speakers bureaus for Acadia, Astra Zeneca, Dey Pharma, EnVivo, Eli Lilly, Forum, Genentech, Janssen, Lundbeck, Merck, Otsuka, PamLabs, Pfizer Israel, Servier, Sunovion and Takeda, and he has received research and/or grant support from Acadia, Alkermes, AssureX, Astra Zeneca, Arbor Pharmaceuticals, Avanir, Axovant, Biogen, Braeburn Pharmaceuticals, BristolMyer Squibb, Celgene, CeNeRx, Cephalon, Dey, Eli Lilly, EnVivo, Forest, Forum, GenOmind, Glaxo Smith Kline, Intra-Cellular Therapies, ISSWSH, Janssen, JayMac, Jazz, Lundbeck, Merck, Mylan, Neurocrine, Neuronetics, Novartis, Otsuka, PamLabs, Pfizer, Reviva, Roche, Sepracor, Servier, Shire, Sprout, Sunovion, TMS NeuroHealth Centers, Takeda, Teva, Tonix, Vanda, Valeant and Wyeth.
All authors met the ICMJE authorship criteria and are entirely responsible for the scientific content for this paper. Neither honoraria nor payments were made for authorship.
Supplementary Materials.
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1092852919001287.







