The Challenge of ADHD
As the most common neurodevelopmental disorder of childhood, attention-deficit/hyperactivity disorder (ADHD) is characterized by inattention, hyperactivity, and impulsivity. ADHD is associated with an increased risk of academic, social, and family problems, as well as emotional dysregulation in children and increased risk of substance abuse, motor vehicle/traffic accidents, and teenage pregnancy in adolescents.Reference Mattingly and Anderson 1 ADHD that persists into adulthood has been associated with lower occupational and economic performance, psychiatric comorbidity (eg, depression, substance abuse, emotional lability, and anxiety disorders), increased rates of marital problems/divorce, and more negative assessments of self-worth and self-esteem.Reference Mattingly and Anderson 1 Although ADHD-related impairments during school and work are generally the primary focus of intervention, ADHD behaviors at the beginning and end of the day can be just as impairing as well as stressful and frustrating for affected individuals and their families.Reference Mattingly and Anderson 1
Comorbidities: the rule, not the exception, in ADHD
ADHD rarely occurs in isolation. In the National Survey of Children’s Health, parents reported that 64% of children/adolescents with current ADHD had at least one other mental, emotional, and/or behavioral disorder.Reference Danielson, Bitsko and Ghandour 2 The most common are behavioral/conduct disorder, anxiety problems, depression, autism spectrum disorder, and Tourette syndrome.Reference Danielson, Bitsko and Ghandour 2 The incidence of psychiatric comorbidity in adults with ADHD reportedly approaches 90%, with anxiety, depression, and substance abuse being the most common.Reference McGough, Smalley and McCracken 3
Patients with ADHD and significant comorbid mood or anxiety symptoms are frequently encountered by clinicians. The traditional practice has been to prioritize mood and anxiety problems before ADHD symptoms. Recent findings regarding antidepressant resistance suggest that this approach may need to be reconsidered.Reference Chen, Pan and Hsu 4 The risk of antidepressant resistance was found to be >2-fold higher in patients with major depression and comorbid ADHD vs major depression alone.Reference Chen, Pan and Hsu 4 Concomitant ADHD treatment was associated with a significantly lower risk of antidepressant resistance, leading to the conclusion that prompt and regular treatment of ADHD in dual-diagnosis patients may reduce the risk of antidepressant resistance.Reference Chen, Pan and Hsu 4 Because the functional impairments of ADHD can exacerbate underlying mood disorders, anxiety disorders, and substance abuse, the careful treatment of ADHD may provide a more favorable environment for effective treatment of these psychiatric comorbidities.Reference Mattingly and Anderson 1
Shortcomings of stimulant therapy
While stimulants (ie, methylphenidates, amphetamines) have been the primary pharmacologic therapy in ADHD for decades, they are not suitable in many situations, including the 25% to 30% of patients who do not achieve optimal symptom reduction or do not tolerate stimulants;Reference Connor and Barkley 5 some patients with comorbidities (eg, anxiety, depression, and tics) or conditions (eg, sleep disorders, eating problems) in which stimulant effects are of concern; in patients requiring 24-hour symptomatic coverage; and in situations where there is a risk of nonmedical use, drug diversion, or abuse of these Schedule II (CII) drugs. Because CII drugs have a high potential for abuse and severe psychological or physical dependence, nonmedical use/diversion of stimulants is not a trivial issue, particularly in light of data that nearly 60% of college undergraduates with current prescriptions for stimulants reported having given away or selling their stimulant medication at least once.Reference Gallucci, Martin and Usdan 6 In children, adverse effects of long-term, continuous stimulant use on growth trajectories that may modestly reduce adulthood height and increase weight and body mass index are also cause for concern.Reference Posner, Polanczyk and Sonuga-Barke 7
Nonstimulants currently approved for ADHD by the FDA are limited to atomoxetine (ATX) and extended-release formulations of guanfacine (guanfacine-XR) and clonidine (clonidine-XR). Of these, only ATX is approved for use in adults. This limited scope of nonstimulant options is likely to expand in the next several years since several agents are in clinical development and have successfully completed Phase 2 and/or Phase 3 studies. Each candidate is unique in terms of chemical structure and putative molecular targets (Figure 1; Table 1). Based on these pharmacologic profiles, nonstimulants can be broadly classified as (1) monoamine reuptake (transporter) inhibitors (eg, ATX); (2) receptor modulators (guanfacine-XR, clonidine-XR); and (3) multimodal agents.
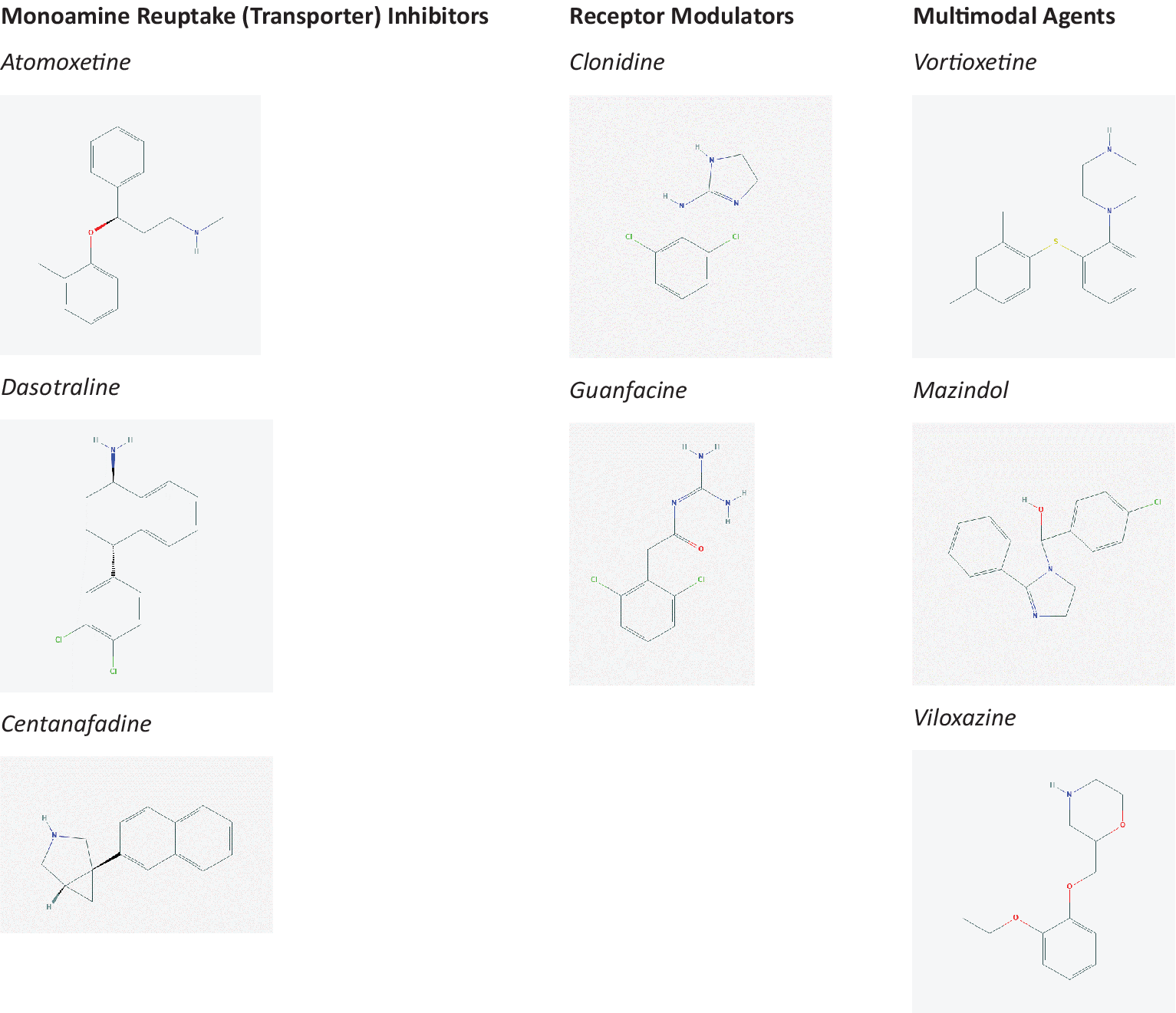
Figure 1. Molecular structure of currently approved and pipeline nonstimulant medications for attention-deficit/hyperactivity disorder.
Table 1. Currently Approved and Pipeline Nonstimulant Medications for Attention-Deficit/Hyperactivity Disorder (ADHD).

Abbreviations: AEs, adverse events; BP, blood pressure; DAT, dopamine transporters; HR, heart rate; MDD, major depressive disorder; NET, norepinephrine transporters; ODD, oppositional defiant disorder; SAD, social anxiety disorder; SERT, serotonin transporters.
We review clinical characteristics of the marketed and experimental alternatives to CII stimulants that have demonstrated efficacy in double-blind, placebo-controlled Phase 2 or Phase 3 studies. Pipeline alternatives to CII stimulants include several monoamine reuptake inhibitors (dasotraline, centanafadine sustained release, OPC-64005) and multimodal nonstimulants (vortioxetine, viloxazine extended-release), as well as a multimodal stimulant (mazindol controlled release) with lower abuse potential (CIV).
Monoamine Reuptake (Transporter) Inhibitors
Atomoxetine
Atomoxetine (Strattera®, Eli Lilly) is a selective noradrenergic reuptake inhibitor with high affinity for presynaptic norepinephrine transporters (NET).Reference Wong, Threlkeld, Best and Bymaster 8 , Reference Bymaster, Katner and Nelson 9 Although ATX was first evaluated in the 1980s as a potential treatment of major depressive disorder (MDD) in adults, development in depression was abandoned due to lack of efficacy. 10
Atomoxetine was the first nonstimulant medication approved by the FDA for the treatment of ADHD based on a series of double-blind, randomized controlled trials (RCTs) in children ≥6 years of age, adolescents, and adults. In a large, comprehensive meta-analysis incorporating data from 24 RCTs in pediatric ADHD, the effect size for total ADHD symptom improvement with ATX was 0.64.Reference Schwartz and Correll 11 Significant differences in ADHD symptoms vs placebo were generally not noted until 4 weeks after treatment was initiated.Reference Schwartz and Correll 11 Atomoxetine was associated with a bimodal response, that is, 45% of patients were much improved at study end, while 40% were considered nonresponders.Reference Schwartz and Correll 11
Several RCTs have examined ATX efficacy and safety in children and/or adolescents with ADHD and specific comorbidities, for example, oppositional defiant disorder (ODD), anxiety disorders, depressive disorders, tic disorders, and autism spectrum disorders.Reference Garnock-Jones and Keating 12 , Reference Clemow, Bushe and Mancini 13 Overall, comorbid disorders did not appear to compromise the efficacy of ATX in reducing ADHD symptoms. Atomoxetine not only did not exacerbate comorbidity symptoms in RCTs,Reference Garnock-Jones and Keating 12 , Reference Clemow, Bushe and Mancini 13 it appeared to have the potential for positive effects on some comorbid symptoms, for example, reducing tic severity and ODD symptoms in children and social anxiety disorder (SAD) in children and adults.Reference Clemow, Bushe and Mancini 13 Improvements in comorbid ODD and anxiety symptoms correlated with improvements in ADHD symptoms.Reference Clemow, Bushe and Mancini 13 Atomoxetine had no significant benefit vs placebo in adults with SAD without comorbid ADHD,Reference Ravindran, Kim, Letamendi and Stein 14 suggesting that previously observed benefits of ATX on comorbid anxiety were mediated by the drug’s effects on ADHD.Reference Koyuncu, Alkin and Tükel 15 Despite the expected improvements in ADHD symptoms, ATX effects on depressive symptoms were no different vs placebo in children and adults with comorbid MDD.Reference Clemow, Bushe and Mancini 13
The most common adverse events (AEs) of ATX in pediatric ADHD RCTs were gastrointestinal symptoms (vomiting, abdominal pain, nausea, and diarrhea), nervous system symptoms (dizziness, headache, somnolence, sedation, and drowsiness), fatigue, and anorexia.Reference Schwartz and Correll 11 In adults, the most common AEs were constipation, dry mouth, nausea, decreased appetite, dizziness, erectile dysfunction, and urinary hesitation. 16 Safety concerns cited in ATX prescribing information include a boxed warning about suicidal ideation in children and adolescents (risk ratio, ATX vs placebo, 2.92Reference Bangs, Tauscher-Wisniewski and Polzer 17 ), as well as warnings about severe liver injury and serious cardiovascular events. 16 Cardiovascular effects of ATX include increases in blood pressure (BP) and heart rate (HR). A comprehensive safety review by the manufacturer found clinically significant increases in HR (≥20 beats per minute [bpm]) and/or BP (≥15-20 mm Hg) in 8% to 12% of children and adolescents.Reference Bushe and Savill 18 Atomoxetine is contraindicated in individuals with severe cardiovascular disorders and should be avoided in children or adolescents with known serious cardiac problems. 16
Dasotraline
Dasotraline (Sunovion) is considered a dual reuptake inhibitor based on its preferential inhibition of dopamine transporters (DAT) and NET and weaker inhibition of serotonin transporters (SERT).Footnote * Reference Hopkins, Sunkaraneni and Skende 20 Like ATX, development of dasotraline in MDD in adults was suspended due to lack of efficacy. 21 , 22 Dasotraline dosed q.d. was subsequently evaluated in a series of RCTs in adults and children with ADHD, starting with a proof-of-concept Phase 2 study in adults treated with 4 mg or 8 mg (estimated DAT receptor occupancy, 56% and 71%, respectively).Reference Koblan, Hopkins and Sarma 23 Only 8 mg dasotraline was superior to placebo (effect size, 0.41) in improving total ADHD symptoms but was not well tolerated (discontinuation due to AEs, 28%) in this exploratory study. Subsequent Phase 3 RCTs in ADHD evaluated 2 to 6 mg dasotraline.
In children and adolescents with ADHD, 4 mg dasotraline was significantly superior to placebo in two RCTs, with an effect size of 0.85 in methylphenidate-responsive patients in a 2-week laboratory classroom studyReference Wigal, Hopkins and Koblan 24 and 0.48 in a 6-week fixed-dose study.Reference Findling, Adler and Spencer 25 In the fixed-dose study, superiority of dasotraline over placebo emerged within the first week. The lower dose of 2 mg dasotraline was not significantly superior to placebo,Reference Findling, Adler and Spencer 25 while evaluation of 6 mg was terminated due to unexpected neuropsychiatric AEs, including hallucinations.Reference Wigal, Hopkins and Koblan 24 In a Phase 3 study in adults, improvement with 4 mg dasotraline failed to show a significant difference vs placebo in improving total ADHD symptoms while the difference with 6 mg dasotraline trended toward significance.Reference Adler, Kollins and Hopkins 26 In a posthoc analysis of pooled data from the two RCTs in adults, improvements in ADHD symptoms were significantly greater with dasotraline (4, 6, and 8 mg/day) vs placebo.Reference Adler, Kollins and Hopkins 26
The most common AEs with 2 and 4 mg dasotraline in children/adolescents were insomnia, irritability, decreased appetite, and decreased weight.Reference Findling, Adler and Spencer 25 Psychosis-related symptoms (hallucinations, delusions) were reported in 5% of children receiving 4 mg and in 10% receiving 6 mg.Reference Wigal, Hopkins and Koblan 24 , Reference Findling, Adler and Spencer 25 Mean BP changes were minimal (≤3 mmHg).Reference Findling, Adler and Spencer 25 No clinically meaningful ECG changes were observed in children. The most common AEs in adults were insomnia, decreased appetite, and dry mouth.
Results of the dasotraline-ADHD clinical development program were reviewed by the FDA, which rejected approval pending additional efficacy and tolerability data. 27 Dasotraline has also been evaluated as a treatment of binge eating disorder in adults. As of this writing, results of this clinical development program were under review by the FDA. 28
Centanafadine
Centanafadine (CTN, Otsuka) is a triple reuptake inhibitor (NET > DAT > SERT) that is being developed as a sustained-release (SR) formulation for b.i.d. dosing in ADHD.Reference Robertson, Shram and Schoedel 29 , Reference Wigal, Wigal and Hobart 30 In a microdialysis study in conscious rats, CTN increased extracellular NE, DA, and serotonin (5-HT) concentrations from baseline by approximately twofold in the prefrontal cortex; striatal extracellular DA was also increased nearly twofold.Reference Robertson, Shram and Schoedel 29 A study in healthy volunteers supported dose-related brain occupancy of CTN-SR on NET, SERT, and DAT.Reference Jennings, Barret and Wisniewski 31 NET occupancy with CTN-SR was equivalent to that of a typical dose of ATX; DAT occupancy was similar to that of a typical long-acting methylphenidate dose. In light of the ability of CTN-SR to increase DA, the drug’s abuse potential was evaluated in an exploratory crossover-design study in healthy recreational stimulant users, comparing an immediate-release (IR) formulation of CTN with d-amphetamine and lisdexamfetamine.Reference Robertson, Shram and Schoedel 29 , Reference Wigal, Wigal and Hobart 30 The subjective effects profile of CTN-IR was different from that of the stimulants. With CTN-IR, aversive effects (dislike) during the initial period when CTN exposure was rapidly increasing were followed by increased “like” mean scores that were numerically lower vs stimulants. Results were interpreted as indicators of low abuse potential for CTN-SR.
Results of two Phase 2 studies have been published.Reference Wigal, Wigal and Hobart 30 In a pilot, single-blind, flexible-dose (target dose, 500 mg/day), 4-week study in adults with ADHD (N = 41), CTN-SR was associated with significant reductions in ADHD symptoms vs baseline.Reference Wigal, Wigal and Hobart 30 A Phase 2b double-blind, crossover study (3-week treatment period; 1-week washout) in 85 adults compared CTN-SR (target dose, 400–800 mg/day) and placebo. CTN-SR was significantly superior to placebo (effect size, 0.66).Reference Wigal, Wigal and Hobart 30 The most frequently reported treatment-related AEs were decreased appetite (24%), headache (23%), and nausea (20%); 10% of subjects discontinued CTN-SR due to AEs. Clinically significant ECG changes were not observed.
Two recently completed Phase 3 double-blind RCTs found significant differences favoring 200 (100 mg b.i.d.) and 400 mg/day (200 mg b.i.d.) CTN-SR over placebo in reducing ADHD symptoms after 6 weeks of treatment in adults. 32 The most common AEs occurring more frequently with CTN-SR vs placebo were decreased appetite, headache, nausea, dry mouth, upper respiratory tract infection, and diarrhea. The incidence of individual AEs did not exceed 7%. 32
Receptor Modulators
Extended-release formulations of the α2 adrenoreceptor agonists guanfacine and clonidine are the only FDA-approved ADHD therapies that have pharmacologic activities seemingly limited to receptor modulation. Guanfacine appears to be selective for postsynaptic α2A receptors; clonidine has higher affinity for presynaptic α2A, α2B, and α2C receptors than for postsynaptic α2A receptors.Reference Arnsten 35 Although modulators of other receptors [eg, nicotinic acid, histamine, gamma-aminobutyric acid (GABA), 5-HT, adenosine A2A] are of growing interest for their potential utility in treating ADHD, clinical trials of these receptor modulators have so far produced mixed results.Reference Childress and Tran 36 None have advanced to Phase 3 trials in patients with ADHD and are therefore unlikely to be clinically available for several years, if at all.
Clonidine-XR (Kapvay®, Shionogi) and guanfacine-XR (Intuniv®, Shire) were approved by the FDA for use not only as monotherapy in children and adolescents (6–17 years old) with ADHD but also as adjuncts to stimulants, which is their primary use in clinical practice.Reference Daughton and Kratochvil 37 Clonidine-XR is dosed b.i.d.; guanfacine-XR is dosed q.d. In pivotal RCTs of clonidine-XR and guanfacine-XR as monotherapy in youth with ADHD, effect sizes ranged from 0.43 to 0.86.Reference Jain, Segal, Kollins and Khayrallah 38 , Reference Huss, Chen and Ludolph 39 Pooled effect sizes were not significantly different for the two drugs.Reference Hirota, Schwartz and Correll 40 In fixed-dose clonidine-XR and guanfacine-XR monotherapy studies, improvements in ADHD symptoms were significantly greater vs placebo by week 1 or 2, with ADHD symptoms typically improving further over the duration of the 8- and 9-week studies.Reference Jain, Segal, Kollins and Khayrallah 38 , Reference Biederman, Melmed and Patel 41 , Reference Sallee, McGough and Wigal 42
Several RCTs of α2-agonist efficacy in ADHD also assessed effects on scores for oppositional behavior and conduct problems in children with or without comorbid ODD or CD. Based on a meta-analysis, evidence was weak that the small effect of clonidine (clonidine-IR and clonidine-XR combined) on oppositionality/conduct scores was clinically important.Reference Pringsheim, Hirsch, Gardner and Gorman 43 Evidence was somewhat stronger for positive effects of guanfacine-XR on oppositional behavior scores.Reference Pringsheim, Hirsch, Gardner and Gorman 43 , Reference Findling, McBurnett, White and Youcha 44 A meta-analysis of α2-agonist RCTs in patients with chronic tic disorders with or without ADHD demonstrated a medium-to-large effect of α2 agonists (clonidine-IR) in studies enrolling patients with tics and ADHD vs no significant benefit in trials excluding patients with ADHD, suggesting a potential benefit in youth with ADHD and comorbid tic disorders.Reference Weisman, Qureshi and Leckman 45
The more common AEs associated with clonidine-XR and guanfacine-XR are sedation/somnolence, fatigue, and gastrointestinal symptoms (nausea, abdominal pain). Consistent with the therapeutic use of α2-agonists as antihypertensives, clonidine-XR and guanfacine-XR can cause dose-related decreases in BP and HR. A small but statistically significant prolongation of QTcF interval was observed with guanfacine-XR vs placebo, although guanfacine-XR does not appear to interfere with cardiac repolarization associated with proarrhythmic drugs.Reference Hirota, Schwartz and Correll 40 , 46 Safety concerns about the cardiovascular effects are reflected in prescribing information, that is, clonidine-XR and guanfacine-XR should be used with caution in patients at risk of hypotension, bradycardia, and, in the case of guanfacine-XR, syncope. 46 , 47 Heart rate and BP should be monitored before and after initiating therapy with these agents, while patients should avoid becoming dehydrated or overheated. Prescribing information for clonidine-XR also warns about the risk of abrupt discontinuation and the risk of allergic reactions in patients with a history of allergic reactions, including contact sensitization, with transdermal clonidine. 47
Multimodal Agents
Multimodal agents are defined here as drugs that combine transporter (eg, NET, SERT, and DAT) modulation/inhibition with receptor modulation (agonist and/or antagonist) activity. Several such agents are being evaluated as potential ADHD treatments.
Vortioxetine
Vortioxetine (VORT, Trintellix®, Lundbeck, and Takeda) is approved for the treatment of MDD in adults and has been suggested to improve cognitive processing speed in depressed individuals. It is a SERT inhibitor, agonist of 5-HT1A, partial agonist of 5-HT1B, and antagonist of 5-HT3, 5-HT1D, and 5-HT7. 48 A Phase 2 proof-of-concept RCT of 10 or 20 mg/day q.d. in adults with ADHD failed to detect a significant difference of VORT vs placebo in improving total ADHD symptoms, although it was significantly superior in reducing patient-rated functional impairment due to ADHD.Reference Biederman, Lindsten and Sluth 49 A post hoc analysis that excluded nonadherent patients (32%) resulted in more pronounced changes from baseline in total ADHD symptom scores for both VORT doses; similar patterns were observed in other parameters. The AE profile in this exploratory study was consistent with the known profile of VORT, with the most common AE being nausea. No additional studies of VORT in ADHD are currently underway.
Mazindol
Immediate-release mazindol (MZD-IR) was marketed for the short-term treatment of obesity in adults but was withdrawn from the market for commercial reasons in the early 2000s.Reference Wigal, Newcorn and Handal 50 As an IR formulation, mazindol is historically considered a weak stimulant (CIV) due to a lower potential for abuse and addiction vs the classical CII stimulants. In more contemporary studies, MZD displays multimodal pharmacologic activities that include NET, DAT, and SERT inhibition (relative affinity, 100:12:2),Reference Zhou 51 as well as modulation of serotonin (5-HT1A, 5-HT7), muscarinic, histamine H1, μ-opioid, and orexin-2 receptors.Reference Wigal, Newcorn and Handal 50
In a pilot, 1-week, open-label study in children with ADHD (N = 21) and MZD-IR (1 mg q.d.) was associated with significant improvement in ADHD scores.Reference Konofal, Ahao and Laouénan 52 Almost all patients (19/21) reported ≥1 AE. The most common AEs were decreased appetite (severe in four patients), drowsiness, intestinal distension, and upper abdominal pain; insomnia was not reported. In a Phase 2 flexible-dose, 6-week RCT in adults with ADHD, mazindol controlled-release (MZD-CR, Nolazol®, NLS Pharmaceutics) was associated with significantly greater improvements in ADHD scores vs placebo (effect size, 1.09).Reference Wigal, Newcorn and Handal 50 The proportion of patients with excellent response (≥50% change from baseline) at 6 weeks was 55% vs 16%. A significant difference favoring MZD-CR was observed at the end of week 1. As expected of an agent previously marketed for short-term weight loss, weight was decreased as early as week 1; mean weight loss was 1.7 kg after 6 weeks. The most common AEs were dry mouth, nausea, fatigue, increased HR, decreased appetite, and constipation. Mean increases of ~3 to 6 mmHg in diastolic and systolic BP (DBP and SBP) and ~7 to 11 bpm in HR were observed. Phase 3 RCTs in ADHD are planned. 53
Viloxazine
Viloxazine as an immediate-release formulation was first marketed as an antidepressant in Europe for more than two decades before being discontinued for commercial reasons. Antidepressant efficacy was established in multiple double-blind RCTs in adults with depressive disorders with/without comorbid anxiety.Reference Pinder, Brogden, Speight and Avery 54 Conducted in the 1970s, these RCTs used tricyclic antidepressants (TCAs) as the active control. In these studies, viloxazine immediate-release (150–300 mg/day) and TCAs improved symptoms of depression and comorbid symptoms such as anxiety.Reference Pinder, Brogden, Speight and Avery 54 , Reference Ban, McEvoy and Wilson 55 Significant improvements in depression scores were generally noted within the first week of treatment with viloxazine immediate-release, indicating an early onset of effect.Reference Pinder, Brogden, Speight and Avery 54
The tolerability/safety profile of viloxazine immediate-release that emerged during more than two decades of use in adults was relatively benign for the therapeutic class. Compared with TCAs, viloxazine immediate-release had a better tolerability/safety profile, particularly with regard to sedation and anticholinergic effects. 53 , Reference Pinder, Brogden, Speight and Avery 54 , Reference Ban, McEvoy and Wilson 55 Viloxazine immediate-release was associated with fewer cardiovascular effects,Reference Thompson and Isaacs 56 including minimal detrimental effects on BP.Reference Bayliss and Duncan 57 Viloxazine immediate-release was not associated with dose-related depression of cardiac contractility in healthy volunteers; symptoms did not include cardiac complications in postmarketing reports of viloxazine immediate-release overdose.Reference Warrington, Padgham and Lader 58
The therapeutic effects of viloxazine in depression were historically attributed to NET inhibition. Early preclinical studies, however, found a more complex pharmacologic profile involving the potentiation of 5-HT without inhibiting SERT.Reference Pinder, Brogden, Speight and Avery 54 In contemporary preclinical studies using more sophisticated methods, extracellular 5-HT concentrations in the prefrontal cortex were increased >5-fold by viloxazine by an unknown mechanism that likely involves 5-HT receptor modulation.Reference Yu, Garcia-Olivares and Candler 59 Prefrontal cortex dopamine and norepinephrine concentrations were also increased >5-fold. Monoamine transmitter concentrations were minimally increased in the nucleus accumbens. In addition to moderate NET inhibition, viloxazine exhibited agonistic activity at 5-HT2C, antagonistic activity at 5-HT2B, and weak antagonistic activity at 5-HT7, α1B, and β2 receptors.Reference Yu, Garcia-Olivares and Candler 59
Double-blind, placebo-controlled RCTs have consistently demonstrated a treatment effect significantly favoring viloxazine over placebo in reducing ADHD symptoms, starting with a Phase 2a proof-of-principle study of viloxazine immediate-release (100 mg t. i.d) in adults.Reference Johnson, Saylor and Brittain 60 A Phase 2b dose-ranging RCT in children found that viloxazine extended-release (SPN-812, Supernus Pharmaceuticals) at dosages of 200, 300, and 400 mg dosed q.d. was significantly superior to placebo in improving ADHD symptoms, with effect sizes of 0.547, 0.596, and 0.623, respectively.Reference Johnson, Liranso and Saylor 61 SPN-812 was subsequently evaluated in four Phase 3 double-blind, placebo-controlled, fixed-dose RCTs in children and adolescents with ADHD. Two studies (NCT032475305Reference Nasser, Liranso and Adewole 62 ; NCT03247543Reference Nasser, Hull and Chowdhry 63 ) were conducted in children 6 to 11 years of age (100 mg, 200 mg, 400 mg); two studies (NCT03247517Reference Nasser, Hull and Chowdhry 64 ; NCT03247556 65 ) were in adolescents 12 to17 years of age (200 mg, 400 mg, 600 mg). Depending on target dose, SPN-812 was titrated over 1 to 3 weeks (starting dose: 100 mg in children; 200 mg in adolescents); the target dose was maintained for at least 5 weeks. Across the four studies (N=1354, intent-to-treat population), subjects were treated with SPN-812 (100 mg, n = 147; 200 mg, n = 359; 400 mg, n = 299; 600 mg, n = 97) or placebo (n = 456). In three Phase 3 studies, subjects consistently displayed greater ADHD symptom improvement with SPN-812 vs placebo. Differences vs placebo in the change from baseline in ADHD-RS-5 Total score at end of study (EOS) were statistically significant with once-daily 100 and 200 mg SPN-812 in one Phase 3 trial conducted in children, and with 200 mg and 400 mg SPN-812 in two Phase 3 trials – one conducted in children and one conducted in adolescents. In the fourth study, the primary endpoint was not met; one of the two (400- and 600-mg/day) doses of SPN-812 (600-mg/day) did not separate from placebo. This was possibly due to a higher placebo response observed in this study, which may have confounded the treatment response. For 100-400 mg SPN-812, symptom improvement was observed as early as the first week of treatment. ADHD symptoms steadily improved over the entire course of double-blind treatment. Core ADHD symptom domains—inattention and hyperactivity/impulsivity—were improved to similar degrees. Parent-assessed outcomes generally mirrored those of investigators. Parents perceived greater ADHD symptom improvement, greater reduction of functional impairment, and less stress with SPN-812 treatment vs placebo. The proportion of patients in the Phase 3 studies with 50% or greater improvement in ADHD-RS-5 Total score compared to baseline (50% responder rate) and proportion of patients “much improved” or “very much improved” per the CGI-I scale were also assessed. The 50% responder rate ranged from 34.2% to 48.2% at EOS; 45.0% to 60.6% of patients were categorized as “much” or “very much improved” per CGI-I scale at EOS. SPN-812 was well tolerated. Discontinuation rate due to treatment-related AEs was <5% for SPN-812 treatment groups within each study. The most common treatment-related AEs in the Phase 3 trials of SPN-812 (≥5% incidence) were somnolence, headache, decreased appetite, fatigue, nausea, and upper abdominal pain. The treatment-related AEs usually emerged within the first month of treatment and most resolved with continued treatment. The most common AEs leading to SPN-812 discontinuation (≥2 subjects) were somnolence (n = 7), fatigue (n = 3), nausea (n = 3), tachycardia (n = 3), decreased appetite (n = 2), and headache (n = 2).
A recently published study evaluated the effect of SPN-812 on QTc interval in healthy adult subjects. The study demonstrated that supratherapeutic (1800 mg q.d.) dose of SPN-812 had no significant effect on cardiac repolarization or other ECG parameters, suggesting that it does not pose a risk of cardiac arrhythmias in healthy adults.Reference Nasser, Faison and Liranso 66 Cardiac AEs in SPN-812 Phase 3 study populations were infrequent, with only two incidences of tachycardia and one incidence of ECG T-wave inversion leading to discontinuation and considered related or possibly related to treatment across all four studies. 62-65 During intensive monitoring of suicidality, suicidal ideation and suicidal behavior were infrequent occurrences, with no incidence of suicidal ideation considered related to treatment and only one incidence of suicidal attempt in an SPN-812 treatment group considered possibly related to treatment. 62-65
The Phase 3 clinical program in pediatric population has been completed.Reference Nasser, Hull and Chaturvedi 67 Results from an ongoing Phase 3 study in adults with ADHD (NCT04016779) are expected in 2021. 68
Why More Options to Schedule II Stimulants Are Needed
We propose several reasons that all clinicians should develop a deeper appreciation of nonstimulants in the treatment of ADHD in both children/adolescents and adults and the need for a broader array of options. First, while stimulants are highly effective in reducing ADHD symptoms, a substantial number of patients either fail to respond and/or have unacceptable side effects when treated with stimulants. In such scenarios, the use of nonstimulants is both appropriate and well supported by various treatment guidelines. Second, stimulants often are less than ideal treatment options in ADHD patients with commonly occurring symptoms such as insomnia, poor appetite, subsyndromic anxiety or depression, and failure to thrive. Nonstimulants are treatment options in such patients—either as monotherapy or as cotherapy with stimulants. In addition, continuous, even coverage of ADHD symptoms is often a clinical necessity. However, even the longest acting stimulant formulation often fails to provide adequate duration of coverage. In such situations, nonstimulants can be useful—again, either as monotherapy or combination therapy. Finally, the potential euphorigenic effects and abuse liability that make CII stimulants medications may negatively impact acceptance and adherence due to complex procedures in renewing a CII medication as well as misinformation, biases, or uncertainty about the use of stimulants to treat ADHD.
With more than 30 CII stimulant preparations commercially available, the field of ADHD is currently limited to just three FDA-approved nonstimulant agents that either modulate the norepinephrine reuptake pump or stimulate norepinephrine α2 receptors.Reference Mattingly, Wilson, Ugarte and Glaser 69 Only one (ATX) is approved for use in adults. Atomoxetine has inconsistent and delayed efficacy, with a bimodal distribution of response, and carries significant warnings involving suicidal ideation, liver injury, and cardiovascular effects. The use of clonidine-XR and guanfacine-XR is limited by sedation and somnolence, particularly when used as monotherapy, while safety concerns include cardiovascular warnings, such as hypotension, bradycardia, and the risk of syncope in the case of guanfacine-XR.
In light of the complex neurobiology of ADHD, novel nonstimulant options are needed that may be more suited to the diverse needs of pediatric and adult patients with ADHD and its associated comorbidities, including patients with comorbid psychiatric disorders such as depression or anxiety. Several promising alternatives to CII stimulants with different mechanisms of action are in clinical development, with one such agent currently being reviewed by the FDA. These new agents may be of benefit in ADHD patients in whom treatment has been limited by comorbidities that make stimulants inappropriate as first-line therapy or have inadequate response or side effects with currently available treatment options. Finally, the concept of ADHD symptom remission is gathering more traction in both psychiatry and pediatrics. If clinicians are to accept this as a “gold standard” for all patients with ADHD, then a deeper appreciation of the strengths and weaknesses of both simulants and nonstimulants is necessary.
Conclusions
ADHD medication development has focused on engineering novel systems for CII stimulants—liquids, capsules, sprinkles, chewables, and prodrugs—that have different pharmacokinetics due to different stimulant formulation release profiles. In contrast, current development of novel medications is exploring new molecular entities with different structures and molecular targets. These molecules differ markedly from the older agents by targeting reuptake inhibition of multiple monoamines, modulation of receptors other than noradrenergic α2 neurotransmitter receptors, and multimodal agents that combine reuptake inhibition with direct receptor modulation. Interest in these new agents include their potential for rapid onset of action and a more favorable safety profile. Hopefully, future research will also assess each of these agent’s ability to beneficially influence the diverse clinical needs and comorbidities associated with ADHD, including their effects on mood, sleep, anxiety, combined with the potential for lower abuse liability.
Acknowledgments
Medical writing was provided by Verna L. Ilacqua of ID&A and was funded by Supernus Pharmaceuticals, Inc. The authors are entirely responsible for the content of the article.
Disclosures
A.J. Cutler: Consultant – Acadia, Alfasigma (Pamlab), Alkermes, Allergan, Avanir, Axsome, IntraCellular Therapies, Janssen, Lundbeck, Neurocrine, Novartis, Otsuka, Sage, Shire, Sunovion, Supernus, Takeda, Teva; Speaker/Promotional Honoraria – Acadia, Alfasigma (Pamlab), Alkermes, Allergan, Avanir, Janssen, Lundbeck, Neurocrine, Otsuka, Shire, Sunovion, Takeda, Teva; Research Grants – Acadia, Alkermes, Allergan, Axsome, IntraCellular Therapies, Janssen, Lundbeck, Neurocrine, Novartis, Otsuka, Shire, Sunovion, Supernus, Takeda; Board Member – Neuroscience Education Institute.
G.W. Mattingly: Consultant – Akili, Alkermes, Allergan, Axsome, Ironshore, Intracellular, Janssen, Lundbeck, Otsuka, Neos Therapeutics, Purdue, Rhodes, Sage, Sunovion, Takeda, Teva; Research Grants – Akili, Alkermes, Allergan, Axsome, Boehringer, Janssen, Lundbeck, Medgenics, NLS-1 Pharma AG, Otsuka, Reckitt Benckiser, Roche, Sage, Sunovion, Supernus, Takeda, Teva; Speaker/Promotional Honoraria – Alkermes, Allergan, Ironshore, Janssen, Lundbeck, Otsuka, Sunovion, Takeda.
R. Jain: Consultant – Addrenex, Allergan, Avanir, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Shionogi, Shire, Sunovion, Supernus, Takeda, Teva; Research Grants –Allergan, AstraZeneca, Lilly, Lundbeck, Otsuka, Pfizer, Shire, Takeda; Advisory Board – Addrenex, Alkermes, Avanir, Forum, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Shionogi, Shire, Sunovion, Supernus, Takeda, Teva; Speaker/Promotional Honoraria – Addrenex, Alkermes, Allergan, Lilly, Lundbeck, Merck, Neos Therapuetics, Otsuka, Pamlab, Pfizer, Rhodes, Shionogi, Shire, Sunovion, Takeda, Tris Pharmaceuticals.
W. O’Neal: Employee of Supernus Pharmaceuticals, Inc.




