Implications
Keel bone damage (KBD) is an important welfare problem in laying hens and may cause economic losses. Our cross-sectional study on European organic layer farms identified aviary systems, absence of natural daylight in the hen house, higher percentages of underweight hens and a higher laying performance as possible risk factors of KBD. Based on these findings we recommend that aviary design should be improved. Adequate housing and lighting should enhance hen orientation and movement in the system. Hens should be fed to meet breeder weight standards. Relationships between feed management, laying performance and KBD should be further investigated.
Introduction
Over the last decade, evidence is accumulating that KBD in laying hens constitutes a serious welfare problem in commercial egg production systems. Although non-cage and free-range systems decrease the risk of inactivity osteoporosis (Rodenburg et al., Reference Rodenburg, Tuyttens, de Reu, Herman, Zoons and Sonck2008; Wilkins et al., Reference Wilkins, McKinstry, Avery, Knowles, Brown, Tarlton and Nicol2011), it has become apparent that prevalence of KBD associated to collisions with housing structures or other environmental impact may be very high, especially in aviaries (e.g. Stratmann et al., Reference Stratmann, Fröhlich, Gebhardt-Henrich, Harlander-Matauschek, Würbel and Toscano2015a). Reported average flock prevalences range from about 12% fractures and 14% deviations in Denmark, with no differences between organic and conventional hens (Riber and Hinrichsen, Reference Riber and Hinrichsen2016) to about 83% fractures and 59% deviations in conventional Belgian flocks (Heerkens et al., Reference Heerkens, Delezie, Rodenburg, Kempen, Zoons, Ampe and Tuyttens2016). Staack et al. (Reference Staack, Gruber, Keppler, Zaludik, Niebuhr and Knierim2009) similarly did not find prevalence differences between organic and conventional flocks in Austria and Germany (28% v. 27% birds with deviations or fractures). In another study in Dutch organic egg productions systems, the average flock prevalence of keel bone deviations or fractures was 21% (Bestman and Wagenaar, Reference Bestman and Wagenaar2014).
Keel bone fractures cause chronic pain (Nasr et al., Reference Nasr, Nicol and Murrell2012a and Reference Nasr, Brownea, Caplena, Hothersalla, Murrell and Nicol2013a) and can negatively affect economic performance (Nasr et al., Reference Nasr, Murrell, Wilkins and Nicol2012b). However, the number of scientific studies, especially epidemiological research, on potential risk factors for KBD is still limited. Apparently, this welfare problem has a multifactorial origin: the main underlying problem has been suggested to be a progressive osteoporosis, starting after the onset of sexual maturity where a switching from structural to medullary bone formation occurs. Both bone types are resorbed as a calcium source, leading to a gradual decline of the proportion of the stronger structural bones (Fleming, Reference Fleming2008). In addition, influencing environmental factors for KBD have been identified, such as floor v. aviary systems (e.g. Riber and Hinrichsen, Reference Riber and Hinrichsen2016), perch height (Staack et al., Reference Staack, Gruber, Keppler, Zaludik, Niebuhr and Knierim2009; Wilkins et al., Reference Wilkins, McKinstry, Avery, Knowles, Brown, Tarlton and Nicol2011) and material (Käppeli et al., Reference Käppeli, Gebhardt-Hinrich, Fröhlich, Pflug, Schäublin and Stoffel2011; Stratmann et al., Reference Stratmann, Fröhlich, Harlander-Matauschek, Schrader, Toscano, Würbel and Gebhardt-Henrich2015b) or stocking density (Staack et al., Reference Staack, Gruber, Keppler, Zaludik, Niebuhr and Knierim2009). Moreover, important feeding factors are in particular calcium supply (Fleming, Reference Fleming2008) and supplementation of n-3 fatty acids (Tarlton et al., Reference Tarlton, Wilkins, Toscano, Aver and Knott2013). In terms of the birds’ preconditions, genetics (e.g. Käppeli et al., Reference Käppeli, Gebhardt-Hinrich, Fröhlich, Pflug, Schäublin and Stoffel2011; Heerkens et al., Reference Heerkens, Delezie, Rodenburg, Kempen, Zoons, Ampe and Tuyttens2016; Riber and Hinrichsen, Reference Riber and Hinrichsen2016) and foot health (Gebhardt-Henrich and Fröhlich, Reference Gebhardt-Henrich and Fröhlich2015), were found to be influential. However, other suggested associated factors have to date not been confirmed, namely perch form (Donaldson et al., Reference Donaldson, Ball and O’Connell2012; Bestman and Wagenaar, Reference Bestman and Wagenaar2014), flock size (Wilkins et al., Reference Wilkins, Pope, Leeb, Glen, Phillips, Zimmerman, Nicol and Brown2005; Nicol et al., Reference Nicol, Brown, Glen, Pope, Short, Warriss, Zimmerman and Wilkins2006), access to free range (Sherwin et al., Reference Sherwin, Richards and Nicol2010; Heerkens et al., Reference Heerkens, Delezie, Rodenburg, Kempen, Zoons, Ampe and Tuyttens2016), vitamin D supplementation (Käppeli et al., Reference Käppeli, Gebhardt-Hinrich, Fröhlich, Pflug, Schäublin and Stoffel2011), BW (Fleming et al., Reference Fleming, McCormack, McTeir and Whitehead2004) or BW uniformity (Petrik et al., Reference Petrik, Guerin and Widowski2015).
Many farmers are presumably not yet fully aware of the extent of this welfare problem, since it is not easily visible from the outside, nor related to conspicuous behavioural or performance changes. The aim of this study was to identify potential factors of housing and management associated with KBD in European organic production systems.
Material and methods
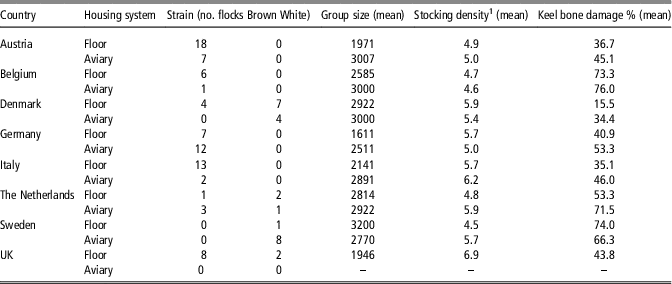
Epidemiological data from 107 organic layer flocks across eight European countries (Austria, Belgium, Denmark, Germany, Italy, the Netherlands, Sweden and the United Kingdom), collected within the CORE Organic II project ‘HealthyHens’ between February 2012 and March 2014, were available for analyses. It was the aim to cover a range of systems that are typical for the majority of organic egg production in each country. Therefore, only farms with stationary housing or mobile houses relocated only after the laying period, as well as farms with at least 500 hen places were included (see Table 1). Spatial distribution of study farms within countries was partly limited due to driving time restrictions. Moreover, inclusion of farmers depended on their willingness to participate in the study. Farms purchasing compound feed were prioritised in order to use feed declarations as an information source.
Table 1 Distribution of strains, group sizes, stocking densities and keel bone damage prevalences of laying hen flocks over the eight countries sorted by housing system floor or aviary

1 Number of hens per m² usable area including the covered veranda (if present).
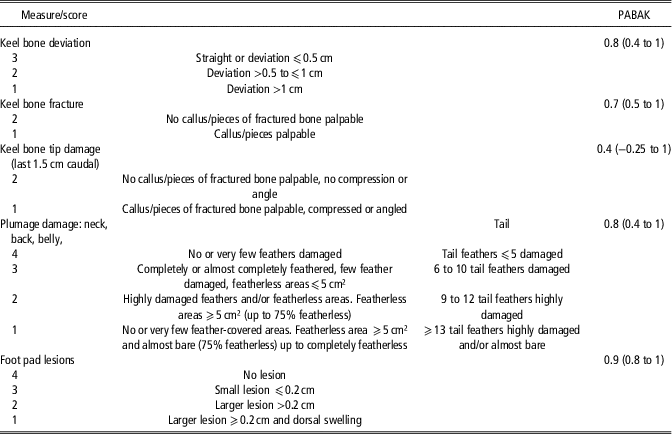
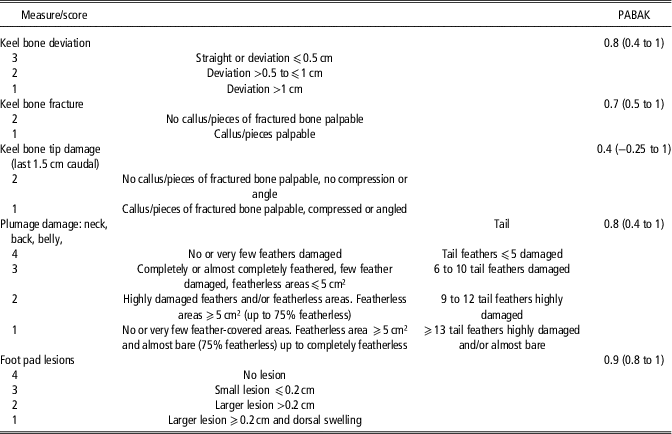
Per country one to two assessors with different experience in the assessment of resource and animal based measures recorded the data. Before the start of the farm visits, all 12 assessors were trained regarding all measurements. Inter-assessor agreement was tested for animal-based measures (Table 2).
Table 2 Scoring systems for keel bone damage, plumage damage and foot pad lesions of laying hens and achieved pairwise inter-assessor agreement between 12 assessors
PABAK=prevalence-adjusted bias-adjusted κ.
Median (range).
Animal-based measurements
Keel bone damage, hen weight, plumage damage and foot pad lesions (FPL) of at least 50 hens/flock (in nine cases 100 to 120 hens/flock) were scored at the age of 52 to 73 weeks (mean 62.4). The hens were caught in different litter and slat areas, aiming to cover the whole hen house and to achieve a random sample. Assessed hens were marked on their leg in order to exclude double scoring. Keel bones were scored by visual inspection and palpation with respect to keel bone deviations and fractures (Table 2). For data analysis both categories were afterwards merged to KBD absent (score 3 for deviations and score 2 for fractures) or present (all other scores and combinations). Assessments of the keel bone tip (last 1.5 cm of the keel bone) were excluded from analyses in general, because of poor inter-assessor agreement. Hens were weighed and results compared with breeder weight standards for the specific genetic strain and age. If weighing less than 90% of the recommended weight, a hen was considered underweight. The percentage of underweight hens was calculated per flock. Four-point scales were used for the assessment of plumage condition (Bestman et al., Reference Bestman, Verwer, Brenninkmeyer, Willett, Hinrichsen, Smajlhodzic, Heerkens, Gunnarsson and Ferrante2017) and FPL (Table 2). Foot pad lesions were scored according to Tauson et al. (Reference Tauson, Kjaer, Maria, Cepero and Holm2005) and comprised scabs, ulcerations of the foot pad and visible swellings of the foot. Plumage condition per flock was expressed as mean feather score, calculated from the individual average scores of each bird, while the flock status regarding FPL was based on the percentage of hens per flock with a score <4.
Use of the free range was estimated at an age of 30 to 40 weeks (peak of lay) as well as 52 to 73 weeks (towards end of lay) by three instantaneous scan samples. Thus, each flock was assessed in spring/summer as well as autumn/winter. Numbers of birds in three different zones (0 to 20 m, 20 to 40 m and >40 distance from pop holes) were counted, starting at 0445, 0300 and 0145 h before sunset, irrespective of the time the lights went off in the barn. The variable ‘percentage of hens counted in the outdoor run’ was the mean of the six scans.
Further data recording
Management data were collected by an interview using a standardised questionnaire (included items see Tables 3 and 4). Housing conditions regarding hen house, covered veranda and free range area were recorded through inspection and measurement. Information on feed composition originated either from feed declarations in case of compound feed, or for on-farm-mixed feed from laboratory analyses using NIRS or Weender analysis.
Table 3 Range (minimum, maximum), mean and median of potential metric risk factors regarding keel bone damage in laying hens for the total and the final sample
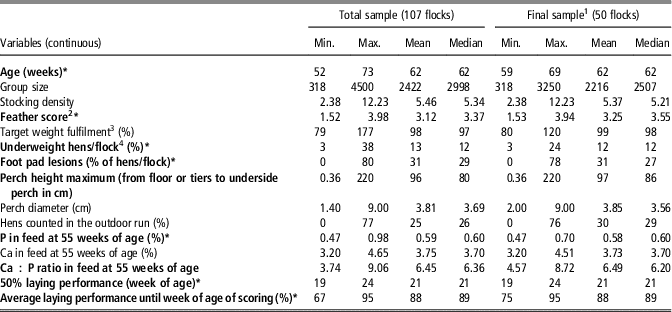
Factors in bold were pre-selected from the total sample, those with * from the final sample for inclusion in the multiple regression modelling.
1 Only flocks without missing values for the pre-selected variables.
2 Scores see Table 1.
3 Hen weight/target weight for the specific week of age according to guidelines of the breeding company×100.
4 Percentage of hens with ⩾11% underweight in comparison to breeder weight standards for the specific week of age.
Table 4 Distribution of flocks with regard to potential dichotomous risk factors concerning keel bone damage in laying hens for the total and the final sample
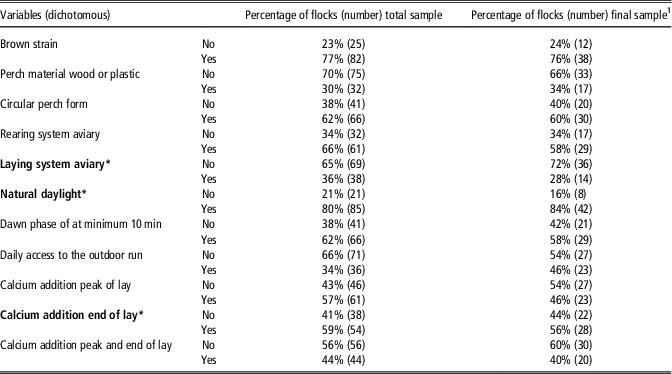
Factors in bold were pre-selected from the total sample, those with * from the final sample for inclusion in the multiple regression modelling.
1 Only flocks without missing values for the pre-selected variables.
Statistical analysis
The dependent variable was KBD prevalence per flock in per cent. The independent variables, potential associated factors regarding KBD, were based on scientific literature and the authors’ practical experience. Parameters with more than 5% missing values and categorical variables with less than 10% of values per category were excluded from analyses. In order to handle missing values without losing too many cases, univariable pre-selection of variables was carried out in two steps, each time using Spearman correlation analysis for continuous variables and Mann–Whitney U test for dichotomous variables. Variables with a P-value below 0.1 in the first tests (total sample, descriptive data see Tables 3 and 4) were analysed again with Spearman correlation or Mann–Whitney U tests after excluding all flocks with a missing value for one of the variables of interest (final sample, descriptive data see Tables 3 and 4). The variables which once again had a P-value below 0.1 were further analysed by forward stepwise selection in the linear regression model. Model diagnostics were carried out graphically, for evaluating linearity of variables and homogeneity of variance by scatter plots of studentised residuals and unstandardised predicted values. Normal distribution of residuals was evaluated with histograms and p–p plot. The absence of strong collinearity between factors was checked using collinearity statistics and variance inflation factor, where values should not be >5 (Menard, Reference Menard1995). The absence of influential data points were checked by Cooks‘ distance (⩽1.0; Cohen et al., Reference Cohen, Cohen, West and Aiken2003). The Bayesian information criterion was a criterion for model selection. All statistics were carried out using the software SPSS (version 24).
Results
Flocks
The average group size in aviary systems was 2785 (min. 1000, max. 3200 hens), in floor systems 2222 (min. 318, max. 4500 hens). Table 1 shows the distribution of aviary and floor systems, brown and white layers, group sizes and stocking densities for the eight countries. Protein content in the feed rations at week 55 of the hens’ life varied from 14.6% to 22.2% (n=91), with energy contents between 10.2 and 12.8 MJ of metabolisable energy per kilogram of dry matter (MJ ME/kg) (n=54) and crude fibre contents from 3.0% to 8.5% (n=84). Keel bone damage prevalence values ranged from 3% to 88% affected hens per flock (mean 44.5%). Due to missing values for different variables in the data set, the final data set for the regression model comprised 50 independent flocks, with 2 to 19 flocks per country.
Variable pre-selection
From the 26 variables offered for pre-selection (Tables 3 and 4), 12 were selected in the first step from the total sample. In the final sample (after exclusion of flocks with missing values), nine variables remained and entered the multivariable analysis (Table 5, marked in bold).
Table 5 Pre-selection of variables potentially related to keel bone damage in laying hens by Spearman correlation analysis and Mann–Whitney U test in the total and final sample (P <0.1)

Variables in bold were included in the regression modelling.
r s =Spearman correlation value, N=number of observations, z=score.
Multiple linear regression model
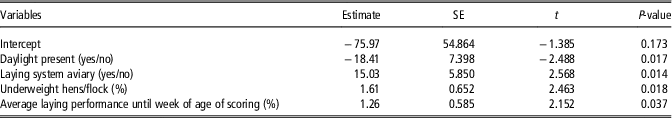
The significant final model (F(4, 45)=6.708, P<0.001, N=50) explained about 32% of the variation in KBD prevalences between flocks and contained the four factors natural daylight in the house (yes/no), aviary v. floor system, the proportion of underweight hens and average laying performance in percent (Table 6). This means that a higher proportion of hens that suffered KBD was seen in case of absence of natural daylight, in aviary systems, when there were more hens with underweight and with higher laying performance.
Table 6 Final linear regression model regarding keel bone damage in laying hens in %

R 2=coefficient of determination; t=hypothesis test statistic.
R 2=0.374, R 2 adjusted=0.318, F (4, 45)=6.708, P⩽0.001; all variance inflation factors⩽1.13; N=50.
Discussion
The ‘HealthyHens’ project focused on the main challenges for organic laying hen farms regarding animal welfare, particularly health, one aspect being KBD. Within the 107 flocks there was no flock without KBD. The lowest prevalence was 3% and the highest 88% affected hens/flock. The high percentage of affected birds as well as the large range of KBD prevalences was similar to those found in other studies (e.g. Rodenburg et al., Reference Rodenburg, Tuyttens, de Reu, Herman, Zoons and Sonck2008; Wilkins et al., Reference Wilkins, McKinstry, Avery, Knowles, Brown, Tarlton and Nicol2011).
The most common techniques to assess KBD are palpation and visual inspection (e.g. Gebhardt-Henrich and Fröhlich, Reference Gebhardt-Henrich and Fröhlich2015; Heerkens et al., Reference Heerkens, Delezie, Rodenburg, Kempen, Zoons, Ampe and Tuyttens2016; Riber and Hinrichsen, Reference Riber and Hinrichsen2016). The presence of callus material (palpable at the ventral and lateral surfaces of the keel), sharp bends or fragmented bone segments indicate fractures, and mere aberrances from the straight axis between the caudal and cranial points of the keel or indentations are commonly termed deviations (Casey-Trott et al., Reference Casey-Trott, Heerkens, Petrik, Regmi, Schrader, Toscano and Widowski2015). In agreement with the recommendations of Casey-Trott et al. (Reference Casey-Trott, Heerkens, Petrik, Regmi, Schrader, Toscano and Widowski2015) we decided to pool different severity grades for KBD into a binary system. Moreover, we summarised fractures and deviations to KBD in general, as the distinction between them is not always clear-cut. Scholz et al. (Reference Scholz, Rönchen, Hamann, Hewicker-Trautwein and Distl2008) histologically found indications for fractures in all cases of severe keel bone deviations, in most cases (90%) of moderate deviations and even in about 50% of slight deviations. Thus, for the final binary scores used in this study, inter-assessor reliability was likely higher than achieved PABAK-values reflect. Poor inter-assessor agreement was, however, reached for the keel bone tip. Consequently, due to its exclusion from further analyses, the resulting associated factors only relate to damage at the main area of the keel.
Including eight EU member states in the study, leaded to a high number (12) of involved assessors with varying prior experience in the assessments. However, the joint training led to acceptable to very good inter-assessor agreement regarding the animal-based individual scoring. The average level of agreement among assessors was comparable to that found in other studies (e.g. Petrik et al., Reference Petrik, Guerin and Widowski2013). It was not feasible to extend the testing of inter-assessor agreement to housing measures, although joint training took place, because it would have required a large number of test farms. Consequently, an assessor bias cannot completely be excluded, although the risk concerning housing and management measures is likely smaller than for animal-based measures. Based on earlier projects and the joint training, definitions of each measure were laid down as precisely as possible. In cases of doubt, data were not used which contributed to a relatively large number of missing values. This partly aggravated the problem of an unbalanced study design with uneven numbers of farms per country owing to differing national funding. Moreover, typical organic husbandry conditions partly differed between countries. For example, aviaries were rare in the United Kingdom and therefore not present in the sample. Similarly, in our data set no brown layers were included in Sweden, nor white layers in Austria, Belgium, Germany and Italy. We refrained from using country as a covariate in the data analysis because it might have overruled such factors that were rather homogenous within country. Therefore, confounding with certain additional effects present in individual countries, such as use of certain feed ingredients or the already mentioned assessor bias, cannot completely be excluded. However, we expect only minor such effects as we minimised assessor bias by training and reliability testing. We furthermore checked that results regarding housing system were still consistent after excluding the data from the United Kingdom.
Absence of natural daylight
According to the regression model, farms without natural daylight in the hen house had about 18% points more hens with KBD than those with natural daylight. The EU regulation on organic farming (EU, 2008) requires the provision of daylight in the house. However, in ten of the 21 houses without daylight, windows were shaded because of feather pecking or cannibalism problems, reasons concerning the other houses are unknown. Thus, there are several possible mechanisms underlying the association found. In flocks with feather pecking or cannibalism problems, the disturbance in the flock may contribute to more escape behaviour and accidents in the house. Poor feathering may at the same time impair flying abilities of the hens. Riber and Hinrichsen (Reference Riber and Hinrichsen2017) reported an association between plumage and KBD, with hens with poor plumage being more likely to have KBD (31.5% v. 22.2%). The small but significant univariable correlation between feather score and KBD in our pre-selection from the total data set points into this direction, too. However, an effect was not conspicuous in the final sample anymore, so that the factor was not selected for the final modelling. Another possible explanation for the association between natural daylight and KBD may be lower light levels in houses without daylight, with negative effects on the birds’ jumping and landing behaviour (Taylor et al., Reference Taylor, Scott and Rose2003). Unfortunately, light intensities were not measured in the current study. It could also be that the part of UV-A radiation (UV-radiation) that penetrates windows, increases brightness perception in the birds (Kämmerling et al., Reference Kämmerling, Döhring, Arndt and Andersson2017). A last option might be positive effects of UV-radiation on bone strength, as found by Fleming (Reference Fleming2008) in UV-treated broilers. However, it appears less likely that this mechanism was involved in our study, because on one hand only small percentages of UV-radiation penetrates windows; and on the other, organic birds have access to outside. However, if birds are more active under daylight conditions, this might indirectly improve bone structure (Whitehead, Reference Whitehead2004; Fleming, Reference Fleming2008).
Aviary systems
In line with earlier studies, we found aviary systems to be associated with increased KBD prevalences compared to floor systems (e.g. Rodenburg et al., Reference Rodenburg, Tuyttens, de Reu, Herman, Zoons and Sonck2008; Riber and Hinrichsen, Reference Riber and Hinrichsen2016; for raised perches, Wilkins et al., Reference Wilkins, McKinstry, Avery, Knowles, Brown, Tarlton and Nicol2011), while Bestman and Wagenaar (Reference Bestman and Wagenaar2014) found no relation between KBD and floor or aviary housing systems. According to our regression model, farms with aviary systems had about 15% points more hens with KBD than those with floor systems. However, within aviaries there may be considerable variation. Other studies have found that certain design aspects are negatively associated with KBD prevalence. These comprise among others type of system (portal instead of row aviaries, Heerkens et al., Reference Heerkens, Delezie, Rodenburg, Kempen, Zoons, Ampe and Tuyttens2016), tier flooring material (plastic slats instead of wire mesh; Heerkens et al., Reference Heerkens, Delezie, Rodenburg, Kempen, Zoons, Ampe and Tuyttens2016), adding of ramps (Stratmann et al., Reference Stratmann, Fröhlich, Gebhardt-Henrich, Harlander-Matauschek, Würbel and Toscano2015a), provision of plastic instead of metal perches (Käppeli et al., Reference Käppeli, Gebhardt-Hinrich, Fröhlich, Pflug, Schäublin and Stoffel2011), provision of soft perches (Stratmann et al., Reference Stratmann, Fröhlich, Harlander-Matauschek, Schrader, Toscano, Würbel and Gebhardt-Henrich2015b) or lower maximum perch heights above both slats and litter (Wilkins et al., Reference Wilkins, McKinstry, Avery, Knowles, Brown, Tarlton and Nicol2011). In our data set (including more floor than aviary systems) we could not detect any significant influences of perch height, form or softness. Possibly, they were confounded with other aspects of the housing system. Nevertheless, also a number of other studies did not find significant effects of round v. rectangular perches (Donaldson et al., Reference Donaldson, Ball and O’Connell2012; Bestman and Wagenaar, Reference Bestman and Wagenaar2014), of wooden v. plastic or metal perches (Bestman and Wagenaar, Reference Bestman and Wagenaar2014), or three-dimensional perch positioning (Donaldson et al., Reference Donaldson, Ball and O’Connell2012). In general, complex systems such as aviaries make it difficult to record meaningful measures, such as perch height or angles between different tiers, because of the multitude of these measures in different areas of the aviary and interactions with other factors such as type and shape of the different structures. Thus, the investigated systems might have been too heterogeneous to detect minor design effects.
Underweight and high laying performance
The regression model revealed an association between KBD and BW as well as laying performance. Keel bone damage prevalence increased about 1.6% points with each percent point more of underweight hens in the flock and 1.3% points with every additional per cent point of laying performance until the week of scoring. At the same time, we found no association with target weight fulfilment (hen weight/target weight for the specific week of age according to guidelines of the breeding company×100). The percentage of birds with insufficient weight may better reflect the adequacy of nutrient supply. However, no conclusions about causal relationship can be drawn from the present epidemiological study: on the one hand there is the possibility that hens with painful KBD were feeding less, although this has not been confirmed experimentally (Nasr et al., Reference Nasr, Murrell, Wilkins and Nicol2012b and Reference Nasr, Murrell and Nicol2013b). On the other hand, fracture healing processes may require energy that leads to loss of BW. Another explanation for the identified relation would be a protective effect of an adequate nutritious state, by better cover of the keel bone by muscles or by better nutrient supply relevant for the maintenance of bone structure. Our finding that flocks with a higher laying performance had higher KBD prevalence may point into the same direction of a higher risk of inadequate nutrition, but the low variance inflation in the regression model indicates that prevalence of underweight hens and laying performance were independently related to KBD prevalence. Although it has been suggested that high egg production increases osteoporosis, previous studies have found no correlation between laying performance and keel bone fractures (Gebardt-Henrich and Fröhlich, Reference Gebhardt-Henrich and Fröhlich2015; Heerkens et al., Reference Heerkens, Delezie, Rodenburg, Kempen, Zoons, Ampe and Tuyttens2016). Whitehead (Reference Whitehead2004) found that a negative correlation between performance and KBD is more distinct at lower performance levels with longer individual periods out of lay in which bone reformation can take place. Indeed, within the laying period Gebardt-Henrich and Fröhlich (Reference Gebhardt-Henrich and Fröhlich2015) found more new fractures occurring during the time when laying rates were highest. However, average laying performance in our investigated flocks was not particularly low, but roughly in line with breeder standards (e.g. Lohmann Tierzucht GmbH, without year). These areas are still rather inconclusive and should be further investigated.
While in the univariable selection we found indications for higher KBD prevalences with lower phosphorus levels in the diet, no calcium addition at end of lay and conforming to findings of Gebhardt-Henrich and Fröhlich (Reference Gebhardt-Henrich and Fröhlich2015), an earlier onset of laying (in this study achievement of 50% laying performance), these factors did not stay in the multivariable regression model. Probably, overruling factors such as percentage of birds with underweight or average laying performance played a role. The moderate explanatory value of the model underlines the multifactorial nature of KBD. More detailed feeding data (e.g. on kind, time and amount of calcium provision), including feeding during the rearing period, should be taken into account in future investigations.
Other factors
We expected that flocks with a higher age would have higher KBD prevalences due to accumulation of impacts on the keel. In contrast, the univariable pre-selection revealed an opposite association which is hard to explain. However, this factor did not contribute to the final model, probably because of the relatively small range of ages of the different flocks.
Gebhardt-Henrich and Fröhlich (Reference Gebhardt-Henrich and Fröhlich2015) found a positive association between bumble foot occurrence and keel bone fractures on individual bird level. In our data there was a low univariable correlation between flock prevalences of FPL and KBD in the expected direction (positive correlation), but FPL did not significantly contribute to the final regression model.
In line with Wilkins et al. (Reference Wilkins, Pope, Leeb, Glen, Phillips, Zimmerman, Nicol and Brown2005) and Nicol et al. (Reference Nicol, Brown, Glen, Pope, Short, Warriss, Zimmerman and Wilkins2006) we could not detect any relation between group size, stocking density and the occurrence of KBD. Conforming with other studies (Sherwin et al., Reference Sherwin, Richards and Nicol2010; Käppeli et al., Reference Käppeli, Gebhardt-Hinrich, Fröhlich, Pflug, Schäublin and Stoffel2011; Heerkens et al., Reference Heerkens, Delezie, Rodenburg, Kempen, Zoons, Ampe and Tuyttens2016), we also found no relation between the percentage of hens using the outdoor run and KBD.
In conclusion, we found aviary systems, absence of natural daylight in the hen house, a higher proportion of underweight birds, as well as a higher laying performance to be positively associated with KBD in organic layers. It is likely that these factors similarly play a role for the presence of KBD in conventional flocks. Thus, in general particular attention should be paid to an adequate housing design and lighting that allows the birds to orient and manoeuvre safely in the system. Furthermore, the feeding management should aim at achieving a sufficient bird live-weight that fulfils breeder weight standards. Relations between laying performance, feed management and KBD should be further investigated.
Acknowledgements
L.J. was financed by the doctoral program ‘Animal welfare in Intensive Livestock Production Systems’. The authors thank the Lower Saxony Ministry for Science and Culture for the financial support. The authors are grateful to Fehim Smajlhodzic, Cynthia Verwer, Jolien Vander Linden, Anne Larsen, Henrik Krogh Andersen, Alice Willet, Valentina Ferrante and Susanna Lolli for data recording and help with the processing. Jan Tind Sørensen, Frank Tuyttens and Stephen Edge contributed as project leaders in their countries. The authors thank Eike Rommelfanger for his statistical advice. The authors furthermore gratefully acknowledge the financial support for this project provided by the CORE Organic II Funding Bodies, being partners of the FP7 ERA-Net project, CORE Organic II (Coordination of European Transnational Research in Organic Food and Farming systems, project no. 249667).
Declaration of interest
None.
Ethics statement
None.
Software and data repository resources
None of the data were deposited in an official repository.