There is increasing evidence, from in vitro and in situ studies, that the excessive consumption of acidic drinks and foods poses a risk to dental hard tissues(Reference Zero1–Reference Phelan and Rees8). Tooth surface is softened in the early stage, and subsequently bulk material is dissolved, layer by layer, from the tooth surface. This type of tooth wear is defined as tooth erosion and is caused by acids (extrinsic and intrinsic) or chelating agents not involving bacterial action. There is a trend towards the increased consumption of acidic drinks and foods. In 2007, the worldwide annual consumption of soft drinks reached 552 billion litres, the equivalent of just under 83 litres/person per year, and this is projected to increase to 95 litres/person per year by 2012. However, the figure had already reached an average of 212 litres/person per year in the USA in 2009(Reference Packer9). To decrease extrinsic erosive tooth wear, the emphasis should be on preventive strategies that mainly aim at reducing the exposure of teeth to potentially erosive agents.
As a prerequisite, it is essential for medical personnel and patients to have a thorough knowledge of the erosive potential of popular dietary substances. In the past several decades, studies investigating the erosive potential of different dietary substances and medications have been performed in different countries(Reference Ehlen, Marshall and Qian3–Reference Phelan and Rees8, Reference Jager, Vieira and Ruben10). A wide range of drinks, foods and medications, such as soft drinks, sports drinks, juices, salad dressings, candies, herbal teas, alcoholic drinks, vinegar, vitamin C tablets etc., were recognised to be associated with the increase in erosion. Normally, soft drinks are mainly composed of filtered water, artificial additives and refined sugar. Thus, they offer limited nutritional benefit, but energy. Sports drinks, which are designed to replenish fluids lost during activity, typically contain water, electrolytes and sugar. Energy drinks are basically soft drinks that contain some forms of vitamins and other chemicals that boost energy for a very short span.
Various chemical properties of a potentially erosive agent, such as pH value, titratable acidity, buffering capacity, the concentrations of Ca, Pi and F, have been identified in the literature to be potentially important in determining the erosive potential(Reference Lussi, Jaggi and Scharer4, Reference Lussi, Jaeggi and Jaeggi-Scharer5, Reference Hara and Zero7, Reference Owens11, Reference Edwards, Creanor and Foye12). However, to the best of our knowledge, no thorough analyses of the effects of a wide range of erosive agents have been undertaken. Buffering capacity is associated with the undissociated acid in a solution, and maintains the H+ concentration and driving force for demineralisation at the site of dissolution(Reference Gray13, Reference Featherstone and Rodgers14). The greater the buffering capacity of the solution, the longer it will take for saliva to neutralise the acid, and as a result the more tooth mineral may be dissolved before a safe pH value is reached and the dissolution ceases. It is important to distinguish buffering capacity from titratable acidity. The latter measures total available H+ over a wide range of pH values, whereas the former is defined at a certain pH value.
The aims of the present in vitro study were twofold: (1) to evaluate the erosive potential of different drinks, foods and medications; (2) to determine those chemical properties that have an impact on the erosive potential.
Materials and methods
Preparation of enamel specimens
From a pool of extracted teeth, six hundred caries-free human premolars with no cracks on the buccal sites as viewed under a stereomicroscope were selected. After the crowns of all teeth were separated from the roots, the buccal sites were ground flat under water-cooling on a LaboPol-21 rotating polishing machine (Struers, Ballerup, Denmark) as follows: groups of five enamel slabs were embedded into one resin disk (Paladur, Bad Homburg, Germany) in two planar parallel molds. Once the hardening process was complete, the thinner mold (200 μm thick) was removed. The outer 200 μm of enamel were ground away with a silicon carbide paper disc of 18 μm grade. Thereafter, the exposed buccal sides of enamel slabs in the thicker mold (7 mm thick) were serially polished on the polishing machine under constant cooling with silicon carbide paper discs of 8 μm grade for 30 s and with 5 μm grade for 1 min. Then, after being taken out of the molds, the embedded resin disks, each containing five enamel slabs, were polished for 1 min with 3 μm diamond abrasive on DP-Mol polishing cloth (Struers). After each polishing step, the resin disks were rinsed and sonicated for 2 min in tap water. These preparation steps wore away 200 μm enamel substance in the centre of the window. Then all the resin disks with embedded enamel slabs were stored in a saturated mineral solution (1·5 mm-CaCl2, 1·0 mm-KH2PO4, 50 mm-NaCl, pH 7·0)(Reference Zero, Rahbek and Fu15).
Tested dietary substances and medications
In the present study, sixty popular drinks, foods and medications in Switzerland were included (Table 1). According to their constituents and applications, these agents were divided into twelve groups: soft drinks, an energy drink, sports drinks, alcoholic drinks, juice, fresh fruit, mineral water, yogurt, tea, coffee, salad dressing and medications. Immediately before the experiment started, the fruits were crushed, and the pulps and seeds were removed by centrifugation; medication tablets and powders were dissolved in tap water according to the suggestions of the manufacturers.
Table 1 Basic information and various chemical parameters of the tested agents, e.g. pH value, titratable acidity to pH 7·0, buffering capacity at the pH value, Ca, Pi, and F concentrations, degree of saturation with respect to hydroxyapatite and fluorapatite*

pK − pI, degree of saturation; HAP, hydroxyapatite; FAP, fluorapatite; NA, not available.
* Titratable acidity, mmol OH-/l to pH 7·0; β, buffering capacity at the pH value; pK − pI with respect to HAP and pK − pI with respect to FAP.
Chemical analysis of tested agents
The pH value and the amount of base needed to raise the pH to 7·0 (titratable acidity) were measured with a titrator (Toledo DL 53, Mettler Toledo, Electrode DG 101-SC, Software: LabX pro, Schwerzenbach, Switzerland). To measure titratable acidity, 10 g of each drink or solution were titrated with 0·5 m-NaOH in steps of 0·02 ml at a temperature of 30°C. The buffering capacity (β) was calculated by using the following equation: β = − ΔC/ΔpH, where ΔC is the amount of base used and ΔpH is the change in pH caused by the addition of the base. In the present study, the buffering capacity at the original pH of the tested products was calculated.
All the tested agents were further analysed for Ca by standard atomic absorption using an atomic absorption spectrometer with an air/acetylene flame. Lanthanum was added to all the products and standards to suppress Pi interference. Total Pi concentration was analysed by the ammonium molybdate method of Chen et al. (Reference Chen, Toribara and Warner16). F concentration was determined using a F ion-specific electrode (Orion 960900, Boston, MA, USA). Before F measurement, all products and standard solutions were mixed with total ionic strength adjustment buffer (TISAB). The concentrations of Ca and Pi are expressed in mmol/l and those of F in mg/l.
The degree of saturation (pK − pI) with respect to hydroxyapatite (HAP) and fluorapatite (FAP) was calculated from the pH and the concentrations of Ca, Pi and F using a computer program(Reference Larsen17). This program assumes a solubility product for HAP of 10− 58·5 and for FAP 10− 59·6(Reference McDowell, Gregory and Brown18, Reference McCann19).
Before the experiment, carbonated drinks were degassed by stirring at room temperature to avoid the adherence of bubbles to the enamel surface, which will affect the chemical analyses and hardness measurements. The concentrations of Ca, Pi and F, the pH and the titratable acidity were measured in duplicate, and for further calculations of buffering capacity and pK − pI, the mean was determined.
Surface hardness measurement
Surface hardness (SH) of the enamel specimens was determined with a Vickers diamond under a pressure of 50 mN for 15 s (Fischerscope HM 2000 XYp; Helmut Fischer, Hünenberg, Switzerland). A total of six baseline indentations were made at intervals of 70 μm. Further indentations next to the previous indentations were made following the experimental procedure. Vickers hardness was calculated from the dimensions of the indentations. The load resolution was ≤ 0·04 mN and the indentation depth was 600 nm for sound enamel and < 1000 nm for most softened specimens. The device allowed fully automatic measurements using a programmable x, y stage. The WIN-HCU software calculated and illustrated SH.
Study design
After polishing the exposed enamel surface of resin disks (five enamel slabs each) with a 3 μm diamond abrasive, six baseline indentations per specimen were made and measured. The mean SH for each resin disk, i.e. the average SH of five enamel samples, was then calculated. According to the SH distribution, two disks, with a total of ten enamel samples, were assigned to one of sixty groups. Thus, the average SH of each pair of disks was similar. Just before the experimental procedures, the resin disks were further polished with a 1 μm diamond abrasive for 1 min (LaboPol-6, DP-Mol Polishing, DP-Stick HQ; Struers, Copenhagen, Denmark), which assured the removal of the possible remnants from storage.
Before the erosive challenge, enamel specimens were immersed in 20 ml of freshly collected human saliva for 3 h to form a salivary pellicle. The saliva, stimulated by paraffin wax (Fluka; Sigma-Aldrich Chemie GmbH, Munich, Germany), was collected in an ice-cooled tube from a single healthy donor at least 1 h after any intake of drink or food(Reference Stiefel20, Reference Wetton, Hughes and West21). She gave informed consent, and saliva collection was performed in accordance with the protocol approved by the University of Bern (Bern, Switzerland). After being carefully rinsed with tap water for 50 s, with deionised water for 10 s and then dried for 5 s with oil-free air, the SH baseline of the samples was measured. Afterwards, the resin disks with five enamel specimens each were individually placed in 60 ml (or g) of the appropriate solution under constant agitation (95 rpm) at 30°C (shaking bath Salvis; Renggli AG, Rotkreuz, Switzerland). After immersion for 2 and 4 min, the resin disks were taken out of the solution, and the SH measurement was performed once again.
Statistics
Wilcoxon's signed rank tests were calculated in an attempt to compare the SH values before and after immersion. The relationship between the changes in SH (ΔSH) within the first 2 min (ΔSH2–0 = SH2 min − SHbaseline) and the second 2 min (ΔSH4–2 = SH4 min − SH2 min) immersion (dependent variables) and pH, buffering capacity, and Ca, Pi and F concentrations (independent variables) was investigated using multiple linear regression (backward selection) analyses. Only variables independent from each other were included. The pK − pI and the titratable acidity were not eligible for inclusion. To assess the bivariate associations between different chemical properties and ΔSH after 2 or 4 min exposure, Spearman's correlation coefficients were used. The statistical calculations were performed using SAS Enterprise Guide 4.1 software. The significance level was set at 0·01 in Wilcoxon's signed rank tests and at 0·05 in multiple linear regression and Spearman's correlation analyses.
Results
Changes in the surface hardness of enamel
In Table 2, Wilcoxon's signed rank tests revealed a significant reduction (P < 0·01) of ΔSH2–0 for soft drinks, sports drinks, the energy drink (Red Bull), juices (except for carrot juice), fruits and salad dressings. Except for Isostar (sports drink) and Thomy French Classic salad dressing, these substances presented a trend towards further decrease in ΔSH4–2. On the contrary, no statistically significant change was found for coffee, most mineral waters, teas and yogurts in both ΔSH2–0 and ΔSH4–2. Exceptions were rose hip tea, forest berries yogurt and Valser Viva Lemon mineral water that had a similar erosive effect as soft drinks. A complicated erosive pattern was observed in the medication and alcoholic drink groups. For example, Alca-C, Alcacyl 500 and Berocca fizzy tablets did not induce a significant decrease in SH, while the reduction was observable within the first 2 min for Aspirine-C fizzy tablet and within the second 2 min for Siccoral, Alka-Seltzer and Fluimucil 200 fizzy tablets. In the alcoholic drink group, by the end of the experiment, Cynar, Carlsberg beer and Montagne red wine did not produce any significant changes in SH of enamel specimens, whereas Eichhof beer demonstrated erosive potential within the second 2 min. It is worth noting that as no adjustment for multiple testing was done, the present results can only be taken into exploratory consideration.
Table 2 Original surface hardness (SHbaseline) of specimens, and the changes within the first 2 min (ΔSH2–0=SH2 min−SHbaseline) and the second 2 min (ΔSH4–2=SH4 min−SH2 min) incubation in different dietary agents and medications
(Mean values with their standard errors)

* Mean values were significantly different in SH within the first 2 min of erosive challenge.
† Mean values were significantly different in SH within the second 2 min of erosive challenge.
Influence of different chemical properties on changes in surface hardness
Table 1 also gives an overview of the chemical properties of all tested agents.
Coffee, teas (except for rose hip tea), mineral waters (except for Valser Viva Lemon mineral water) and some medications (Alcacyl 500, Alka-Seltzer and Aspirine-C fizzy tablets) had the highest pH values, above 5·5. The lowest pH values, varying between 2·4 and 3·3, were mostly found in the soft drinks and the energy drink (Red Bull).
The larger titratable acidity was found for fruits, salad dressings, yogurts as well as for grapefruit and orange juices (>100 mmol/l). The buffering capacity ranged from 2·0 to 200 mmol/l × pH. The highest values were observed for yogurts, fruits (except for orange) and salad dressings (>95 mmol/l × pH), the lowest values for Siccoral, Henniez mineral water, coffee and tea (except for rose hip tea) ( < 3 mmol/l × pH).
Yogurts contained the highest concentrations of Ca (>43 mmol/l) and Pi (>33 mmol/l). Black tea contained the highest concentration of F (1·63 mg/l), whereas F concentration in other agents normally varied between 0 and 1 mg/l.
Many of the test agents under study were undersaturated with respect to both HAP and FAP. Exceptionally, Henniez mineral water, Alcacyl 500 and Alka-Seltzer fizzy tablets, Kiwi and Slimline yogurts, coffee and teas (except for rose hip tea) were supersaturated with respect to both minerals. Valser mineral water, natural and forest berries yogurts, Thomy French Light salad dressing were undersaturated with respect to HAP but supersaturated with respect to FAP.
Table 3 shows the chemical properties with a significant impact on ΔSH after a 2 and 4 min immersion in the multiple linear regression analysis. In this analysis, 52 % of the variation of ΔSH after 2 min immersion and 61 % of the variation after 4 min immersion could be explained by pH, buffering capacity, Ca and F concentrations (P < 0·05).
Table 3 Multiple linear regression analysis of the changes in surface hardness (ΔSH) of all specimens after immersion in all agents for 2 and 4 min*
(β Coefficients)
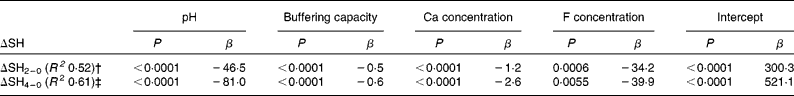
* P values (β: estimate) are listed for those variables with a significant impact on ΔSH.
† ΔSH2–0 = SH2 min − SHbaseline.
‡ ΔSH4–0 = SH4 min − SHbaseline.
There were high bivariate correlations between ΔSH and the pH, the (pK − pI)HAP and (pK − pI)FAP (Table 4). However, the concentrations of Ca, Pi and F, the titratable acidity and the buffering capacity showed small bivariate correlations with ΔSH.
Table 4 Spearman's correlation coefficients: all chemical properties v. the changes in surface hardness (ΔSH) and the respective P values
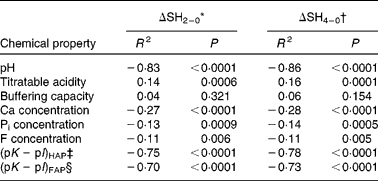
pK − pI, degree of saturation; HAP, hydroxyapatite; FAP, fluorapatite.
* ΔSH2–0 = SH2 min − SHbaseline.
† ΔSH4–0 = SH4 min − SHbaseline.
‡ pK − pI with respect to HAP.
§ pK − pI with respect to FAP.
Discussion
In agreement with previous studies(Reference Lussi, Jaggi and Scharer4, Reference Lussi, Jaeggi and Jaeggi-Scharer5, Reference Larsen and Nyvad22, Reference Jensdottir, Bardow and Holbrook23), the present study indicated that soft drinks, energy drinks (Red Bull), sports drinks, juices, fruits, and some medications and alcoholic drinks caused statistically significant decrease in SH of enamel samples. Yogurts, teas, mineral waters and coffee, except for those that were flavoured with acidic additives, did not have a detrimental effect on enamel SH.
The results highlight the role of acidic additives in increasing erosive capacity of potentially erosive agents. The fruit-based or other acidic flavourings added to ‘plain’ or ‘flat’ drinks and foods, which are intended to stimulate taste, contribute to lower acidity and, consequently, induce erosion. Yogurt is a good example for demonstrating the effect of acidic additives. Natural yogurt caused no erosion in spite of its low pH value (3·91). This can be attributed to its high (pK − pI)HAP resulting from high concentrations of Ca and Pi. The addition of berries (forest berries yogurt) caused a clinically not relevant reduction in SH within the second 2 min exposure. Even though this brand had higher Ca and Pi concentrations than natural yogurt, its pH of 3·77 was too low for it to be supersaturated with respect to HAP. These findings are in accordance with other studies(Reference Caglar, Lussi and Kargul24, Reference Lussi, Jaeggi and Lussi25). Similarly, compared with plain mineral water or tea, flavoured products, such as Valser Viva Lemon mineral water and rose hip tea, had much lower pH and negative (pK − pI)HAP, and hence caused a statistically significant reduction in SH. Moreover, it has been suggested that fruit-based acids might enhance the buffering capacity(Reference Edwards, Creanor and Foye12), which perhaps explains the higher buffering capacity and titratable acidity for the flavoured liquids (Table 1). Therefore, the above-mentioned flavoured products, from the chemical composition point of view, should be classified as soft drinks. Their erosive potential would be expected to be much closer to erosive drinks than to plain products(Reference Parry, Shaw and Arnaud6, Reference Brown, Smith and Shaw26).
The pK − pI with respect to tooth mineral, determined by the pH value and the concentrations of Ca, Pi and F in a solution, is the driving force for mineral dissolution. When (pK − pI)HAP < 0, the solution is undersaturated with respect to HAP, which chemically and structurally resembles natural tooth(Reference Yamagishi, Onuma and Suzuki27). In acidic media, the value of the ion activity product for HAP was a good predictor of enamel lesion(Reference Shellis, Wahab and Heywood28, Reference Shellis29). Therefore, this solution may induce demineralisation of the enamel. When (pK − pI)HAP>0, the solution is supersaturated, so favours remineralisation(Reference Lussi and Jaeggi30). Previous studies have observed that (pK − pI)HAP plays an important role in tooth dissolution. A small change in (pK − pI)HAP might result in a marked difference in the dissolution rate of enamel(Reference Gray13, Reference Margolis, Zhang and Lee31, Reference Tanaka and Kadoma32). As (pK − pI)HAP is dependent on pH and Ca and Pi concentrations, it was not included in the multiple regression analysis. However, there was a negative and strong bivariate correlation between both (pK − pI)HAP and (pK − pI)FAP and ΔSH after both 2 and 4 min. In general, bivariate analyses may be misleading because possible interactions between variables are neglected. Interestingly, the concentrations of Ca, Pi and F alone had a weak correlation with ΔSH[0], whereas the pK − pI defined by the combination of these variables (and the pH) showed a strong correlation.
Many studies have demonstrated that pH is a good predictor of dental erosion: as the pH of the investigated product decreases, there is an increased amount of erosion, independent of the way in which erosion is measured(Reference Larsen and Nyvad22, Reference Mistry and Grenby33). The buffer properties (buffering capacity or titratable acidity) have also been considered to be important(Reference Lussi, Jaeggi and Jaeggi-Scharer5), even more than pH(Reference Grobler, Senekal and Laubscher34, Reference Milosevic35), in predicting the erosive potential because it maintains the H+ concentration available for the interaction with the tooth surface(Reference Zero1). The effect of buffering might, however, vary with pH. Because erosive demineralisation takes place at least partly beneath the enamel surface, buffering capacity may become increasingly important as pH falls, since this is accompanied by an increase in dissolution rate. Consequently, while diffusion may be capable of supplying sufficient H+ ions at higher pH (slow dissolution), increased buffering will be required at lower pH in order to maintain the supply of H+ ions(Reference Shellis, Barbour and Jones36). However, the relative importance of pH and buffering properties could depend on factors such as exposure time and the ratio of the volume of solution to the area of exposed tooth surface. In an in vitro study using a low ratio of solution to specimen area, Jensdottir et al. (Reference Jensdottir, Bardow and Holbrook23) reported a significant correlation between buffer properties, titratable acidity, buffering capacity and tooth tissue dissolution after exposure to selected soft drinks for a long time (24 h), while after a short-term exposure (3 min), erosion was associated with pH but not with titratable acidity(Reference Jensdottir, Holbrook and Nauntofte37). They speculated, therefore, that titratable acidity was the better predictor of erosive potential during longer erosive challenges and pH was better for short challenges. However, Hara & Zero(Reference Hara and Zero7) observed that after 2 h exposure, titratable acidity showed a low-to-moderate correlation with enamel demineralisation, while pH value was the best predictor for erosion. They ascribed this result to the relatively high volume (30 ml) used in their study. Buffering properties are likely to be relatively more important when a low volume of solution is used, as the pH would be raised more easily by mineral dissolution(Reference Hara and Zero7). The dependence of tooth erosion on both pH value and buffering capacity observed in the present study, and the lack of a significant effect of titratable acidity could thus be due to our use of short erosive challenges and an adequate, well-stirred volume of the test product.
The literature is contradictory with regard to the erosive potential of acidic drinks and foods containing F(Reference Lussi, Jaggi and Scharer4, Reference Lussi, Jaeggi and Jaeggi-Scharer5, Reference Larsen and Nyvad22). Previous studies have shown that the erosive capacity of different drinks was significantly and negatively associated with their original F concentration(Reference Lussi, Jaggi and Scharer4, Reference Lussi, Jaeggi and Jaeggi-Scharer5). This observation was confirmed in the present study. In contrast, Larsen & Nyvad(Reference Larsen and Nyvad22) reported that F concentration in eighteen soft drinks had no effect on the depth of tooth erosion. Furthermore, a study by Larsen & Richards(Reference Larsen and Richards38) showed that in drinks with pH above 3, F concentrations reduced the in vitro development of erosion by 28 %; in drinks with pH below 3, erosion was not affected, despite total F concentrations of 20 parts per million and saturation with calcium fluoride. It is worth noting that in those studies, severe acid attacks with surface loss was chosen, while in the present study initial erosion (softening) caused by various agents was assessed.
A higher concentration of the Ca and/or Pi in a solution will increase the pK − pI with respect to dental mineral, so that the presence of suitable concentrations of Ca and Pi may counteract tooth erosion caused by acidic drinks and foods. Some studies have proved that lower levels of enamel demineralisation were found in Ca-containing drinks than in those without Ca(Reference Jensdottir, Bardow and Holbrook23, Reference West, Hughes and Parker39–Reference Hughes, West and Parker41). The relatively higher concentrations of Ca and Pi are most probably responsible for the less erosive effect of Isostar compared with other sports drinks. Isostar does not contain other protective ingredients, such as casein. The results of multiple linear regression analyses indicated a significant relationship between Ca concentration and erosion. However, there was no evidence of a relationship between Pi and tooth erosion. There are four species of inorganic Pi, namely H3PO4, H2PO4−, HPO42 − and PO43 −, in a given solution and their proportions depend on the pH(Reference Dawes42). At the pH of erosive drinks (approximately 2–4), only a minute fraction of the total Pi (of the order of 10− 13) is in the form of PO43 − ions(Reference Dawes42), which are the only important Pi species in the ion activity product of HAP and FAP. Therefore, enormous quantities of Pi are required to raise the degree of saturation of the solution. This may be the reason why Pi is ineffective in the present study.
The formation of a pellicle with human saliva as well as the exposure time scale of a few minutes used in the present study is of particular physiological relevance and clinical interest. First, this exposure time is comparable with clearance time of acids in the mouth(Reference Jensdottir, Bardow and Holbrook23). Second, in the early stage, acids diffuse into the tooth and remove Ca and Pi from the outer few micrometres of hard tissues, forming a demineralised, weakened layer. Remineralisation is possible in this stage, since the remaining enamel can serve as framework in which minerals can be deposited again(Reference Lussi43).
The present in vitro study, however, cannot totally reproduce the clinical conditions, and should only be interpreted as a prediction of the relative erosive potential of a dietary substance or a medication. Erosion is a multifactorial condition, and its occurrence and development depend on many risk and protective factors as well as on their interplay(Reference Lussi, Hellwig and Zero44). In addition to the erosive potential of dietary substances and medications, a variety of factors, for example frequency of acid intake, individual dietary habits (sipping, gulping, frothing or use of a straw)(Reference Zero, Lussi and Lussi45), the physical properties (the adhesiveness and displacement) of these agents(Reference Ireland, McGuinness and Sherriff46), the flow rate, composition and clearing capability of the saliva, may influence the progress of tooth erosion(Reference Hara, Lussi, Zero and Lussi47). However, an investigation of the parameters associated with the erosive potential of dietary substances and medications could act as a significant screening test through which dentists can provide instructional recommendations for patients at high risk of dental erosion. In addition, the present study covered a wide range of tested agents with various chemical and physical properties. Some components in these agents may have an influence on salivary pellicle and thus interfere in the correct assessment of tooth erosion. For example, black tea and red wine have been shown to have a profound effect on in vitro pellicle maturation, causing thickened layers of stained material to build up, which were not readily removed. The mechanism behind this effect was ascribed to the polyphenols contained(Reference Joiner, Muller and Elofsson48). Salivary proline-rich proteins, particularly basic proline-rich proteins, via the proline rings(Reference Williamson49), have a particularly high affinity for dietary polyphenols(Reference Hagerman and Butler50, Reference Lu and Bennick51), as do histatins(Reference Yan and Bennick52, Reference Wroblewski, Muhandiram and Chakrabartty53).
In conclusion, the present study confirmed the erosive potential of a wide range of dietary substances and medications. Tooth erosion had a significant relationship with pH, with buffering capacity, F and Ca concentrations. The degree of saturation with respect to HAP and FAP, illustrating the combined effect of these parameters, showed a high bivariate correlation with tooth erosion.
Acknowledgements
The present study was supported by a grant from the Swiss Society of Odontology (project no. 222-05). A. L. designed the protocol. B. M. conducted the experiments. X. W., R. P. S. and A. L. analysed the data and wrote the manuscript. A. L. had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors reported a conflict of interest. We thank Stefanie Hayoz, Institute of Mathematical Statistics and Actuarial Science, University of Bern, for the statistical analysis, and also Thiago Saads Carvalho, Faculdade de Odontologia da Universidade de São Paulo, for the help in the revision of this manuscript.





