Ageing is a complex process that affects a variety of physiological functions. It is widely accepted that the ageing process attenuates the immune response and results in greater susceptibility to infection or an increased risk of cancer( Reference Gruver, Hudson and Sempowski 1 ). The thymus is a central lymphoid organ responsible for the production of naive T cells, which migrate to the peripheral lymphoid tissues and play a vital role in mediating both cellular and humoral immunity. Age-related involution of the thymus is characterised by a decrease in its size, weight and cellularity, along with structural and functional impairment, culminating in a decrease of cells entering the peripheral T-cell pool. Chronic involution of the thymus is thought to be one of the major factors contributing to the decline of immune function with increasing age( Reference Haynes, Markert and Sempowski 2 , Reference Linton and Dorshkind 3 ).
Vitamin C (VC) is an essential micronutrient that is required for normal metabolism in humans. It is essential for the biosynthesis of collagen and l-carnitine( Reference Rebouche 4 , Reference Li and Schellhorn 5 ), and is also known to be an antioxidant that protects against oxidative stress. The current RDA of VC is 75–90 mg/d in the USA or 100 mg/d in Germany, Switzerland, Austria and Japan( Reference Levine, Wang and Padayatty 6 ). Recently, dietary intake of VC at five to twenty times the RDA has been reported to decrease lipid peroxidation( Reference Huang, Appel and Croft 7 , Reference Nakhostin-Roohi, Babaei and Rahmani-Nia 8 ), oxidative DNA damage in lymphocytes( Reference Panayiotidis and Collins 9 ), total cholesterol( Reference Shidfar, Keshavarz and Jallali 10 ), arterial stiffness( Reference Wilkinson, Megson and MacCallum 11 ) and endothelial damage( Reference Morel, Jesel and Hugel 12 ), as well as prevent the common cold( Reference Van Straten and Josling 13 ). It has also been reported that VC intake improves some immune cell functions( Reference Levine, Dhariwal and Wang 14 – Reference Panush, Delafuente and Katz 16 ); however, the effects of long-term high-dose VC consumption on the number and function of immune cells are poorly understood.
Senescence marker protein-30 (SMP30) is a 34 kDa protein that was originally identified in the rat liver, and its expression has been reported to decrease with age in both sexes( Reference Fujita, Uchida and Maruyama 17 ). SMP30 is also known as regucalcin (RGN) or gluconolactonase (GNL), and it acts as a lactone-hydrolysing enzyme in VC biosynthesis( Reference Maruyama, Ishigami and Kondo 18 ). Recently, Ishigami et al. ( Reference Ishigami, Fujita and Handa 19 ) established SMP30/GNL knockout (SMP30KO) mice and demonstrated that these mice display symptoms of scurvy such as fractures and a rachitic rosary when fed a VC-deficient diet, and die within 135 d( Reference Kondo, Inai and Sato 20 ). Thus, SMP30KO mice are thought to be a more useful animal model for investigating the physiological functions of VC than widely used VC-deficient animals such as osteogenic disorder Shionogi (ODS) rats or guinea pigs.
In the present study, to investigate the effects of long-term high-dose VC intake on age-related changes in immune status, we estimated the daily dietary VC requirement of SMP30KO mice and fed these mice normal- or high-VC diets for 1 year, after which we measured the weights of immune organs and the numbers of immune cells in the peripheral blood, spleen and thymus.
Materials and methods
Animals
SMP30KO mice were generated from C57BL/6 mice by gene targeting, as described previously( Reference Ishigami, Fujita and Handa 19 ). Specific pathogen-free male C57BL/6 (wild-type; WT) mice were purchased from Charles River Japan and were allowed to acclimatise for 7 d before the experiments. Throughout the experiments, mice were housed individually and maintained under specific pathogen-free conditions at our laboratory in a controlled environment (room temperature 23 ± 1°C, relative humidity 55 ± 5 % and photoperiod 12 h light–12 h dark cycle). All experiments were performed with 6- to 7-week-old male SMP30KO mice and 7-week-old male WT mice in accordance with the guidelines of the Animal Care and Use Committee of the House Wellness Foods Corporation.
Experimental design
In a preliminary study, SMP30KO mice were fed a purified diet containing 0·02 % VC (20 mg/kg per d) for 5 months. Then, the plasma ascorbic acid concentration of SMP30KO mice was compared with that of age-matched WT mice to investigate whether our estimated RDA of VC was appropriate. The RDA was estimated by scaling the human dose down to an equivalent dose normalised to the body surface area. In the subsequent main study, SMP30KO mice were randomly allocated to two groups and fed a purified diet (Oriental Yeast Company) containing 0·02 % VC (n 10) or 0·2 % VC (n 11) for 1 year. The composition of the diet was based on the American Institute of Nutrition (AIN)-93M diet( Reference Reeves, Nielsen and Fahey 21 ), as detailed in Table 1. The sample size was determined from the results of a previous study on the effect of melatonin on thymic atrophy( Reference Tian, Zhang and Dai 22 ). An estimated sample size of sixteen mice was based on an expected thymus weight of 26 (sd 4) g in aged C57BL/6 mice, a targeted 25 % restoration of thymus weight, a statistical power of 80 % and a type I error of 5 %. We initially allocated ten to eleven mice to each group to allow for an estimated 20 % dropout rate over the study period. WT C57BL/6 mice (WT group, n 5) were fed a commercial diet (CE-2; CLEA Japan, Inc.) for 1 year and used as aged control mice. Mice were given the aforementioned diets and drinking-water without VC ad libitum throughout the study. After receiving the respective diets for 1 year, the body weight, organ weights and plasma ascorbic acid levels were measured, and peripheral blood leucocytes, splenocytes and thymocytes were prepared for further analyses. Mice were anaesthetised with diethyl ether, and blood samples were taken from the inferior vena cava before mice were killed by exsanguination.
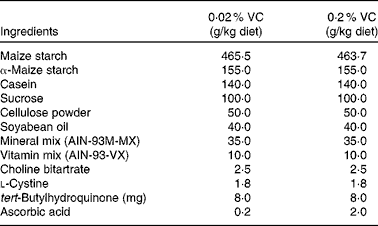
VC, vitamin C; AIN, American Institute of Nutrition.
* The basal diet was based on the AIN-93M diet, which was supplemented with either 0·2 % or 2·0 % ascorbic acid.
† Mice in the wild-type group were fed a powdered CE-2 diet, which is adequate for normal growth. The CE-2 diet contains 89 g moisture, 249 g crude protein, 46 g crude fat, 41 g crude fibre, 66 g crude ash and 510 g N-free extract per kg diet, with an energy content of 14·4 MJ/kg
Measurement of plasma ascorbic acid levels
The plasma level of ascorbic acid was measured by HPLC with electrochemical detection( Reference Umegaki, Nishimuta and Esashi 23 , Reference Margolis and Davis 24 ). The plasma sample was vigorously mixed with an equal volume of 10 % (w/v) metaphosphoric acid containing 0·2 mm-EDTA-2Na, and was then centrifuged at 8000 g for 10 min at 4°C. The supernatant was collected and stored at − 80°C for only 1 week. Before HPLC analysis, the supernatant (10 μl) was vigorously mixed with 40 μl of 10 % (w/v) TCA, and was then centrifuged at 5200 g for 10 s at room temperature. The supernatant (10 μl) thus obtained was diluted with 40 μl of the mobile phase and was then injected into a semi-micro HPLC system (Nanospace SI-2; Shiseido). Chromatographic separation was carried out on a Capcell Pak C18 MG (3·0 × 75 mm, 3 μm; Shiseido) using an isocratic mobile phase (0·1 m-potassium phosphate buffer, pH 2·0). The flow rate was 400 μl/min, the injection volume was 5 μl, and the column oven temperature was 40°C. The electrochemical detector (Model 3016; Shiseido) was operated in the amperometric mode at an oxidation potential of +700 mV.
Preparation of leucocytes, splenocytes and thymocytes
In one set of experiments, blood samples were incubated with cold lysis solution (150 mm-NH4Cl, 10 mm-NaHCO3 and 1·27 mm-EDTA) for 5 min at room temperature. After centrifugation at 250 g for 5 min at 4°C, the lysed erythrocytes were removed. The pellet of leucocytes was washed with PBS containing 2 % fetal bovine serum (FBS) and resuspended in PBS containing 2 % FBS. The spleen or thymus gland was initially minced with scissors and then mashed with the plunger of a syringe, after which the cell suspension was centrifuged at 250 g for 5 min at 4°C. For thymus samples, after removing the supernatant, the pellet of thymocytes was washed and resuspended in PBS containing 2 % FBS. For spleen samples, after removing the supernatant, erythrocytes were lysed by incubation for 5 min at 4°C in lysis solution (17 mm-NH4Cl and 140 mm-Tris–HCl, pH 8·0). Then, an excess of Hank's solution containing 10 % FBS was added and the suspension was centrifuged at 250 g for 5 min at 4°C. Subsequently, the pellet of splenocytes was washed and suspended in PBS containing 2 % FBS. Single-cell suspensions of leucocytes, splenocytes and thymocytes were obtained by passing each suspension through a 70 μm nylon cell strainer (Nippon Becton-Dickinson Company Limited), and were used for cell counting.
Cell counting
Erythrocytes, total splenocytes and total thymocytes were counted with an automated cell counter (CDA-500; Sysmex). The absolute count of each population of leucocytes was analysed by flow cytometry (EPICS XL ADC) using EXPO 32 ADC software (Beckman Coulter, Inc.). In brief, the number of leucocytes was measured using Flow-Count™ (Beckman Coulter, Inc.), and the percentages of lymphocytes, granulocytes and monocytes in the gated population of leucocytes were determined. Then, the absolute count of each cell subpopulation was calculated by multiplying the total number of leucocytes by the percentage of each cell type.
Antibodies
Antibodies used in the present study were as follows: anti-cluster of differentiation (CD)3-fluorescein isothiocyanate (FITC) (clone 145-2C11; Santa Cruz Biotechnology, Inc.); anti-CD4-phycoerythrin (PE) (clone RM4-5; eBioscience, Inc.); anti-CD8–peridinin chlorophyll protein complex (PerCP) (clone 53-6.7; Biolegend); anti-CD4-FITC (clone RM4-5; BD Pharmingen); anti-CD8-FITC (clone 53-6.7; Beckman Coulter, Inc.); anti-CD44-PE (clone KM201; Beckman Coulter, Inc.); anti-CD45RB-PerCP/cyanine5.5 (clone C363-16A; Biolegend); anti-CD16/CD32 (clone 93; Biolegend).
Flow cytometry
Cells were pre-incubated with anti-CD16/CD32 antibodies to block Fc receptors and were then stained with each monoclonal antibody, according to the manufacturer's instructions. After staining, the cells were analysed on a flow cytometer. Thymocytes were stained with anti-CD4-PE and anti-CD8-PerCP antibodies. Double-negative or double-positive thymocytes were defined as CD4−CD8− or CD4+CD8+ thymocytes, respectively, while CD4 or CD8 single-positive (SP) thymocytes were defined as CD4+CD8− or CD4−CD8+ thymocytes, respectively. Leucocytes or splenocytes were stained with anti-CD3-FITC antibodies and with anti-CD4-PE or anti-CD8-PerCP antibodies. In some experiments, these cells were also stained with anti-CD4-FITC or anti-CD8-FITC antibodies, followed by anti-CD44-PE and anti-CD45RB-PerCP/cyanine5.5 antibodies. Among the SP T cells, CD44lowCD45RBhigh cells were defined as naive T cells, and CD44highCD45RBlow cells were defined as memory T cells. The absolute count of each cell subpopulation was calculated by multiplying the total number of thymocytes, splenocytes or lymphocytes by the percentage of each subpopulation.
Statistical analysis
Differences between the 0·02 % VC group and the 0·2 % VC group were assessed by unpaired Student's t test using Statcel 2 software (OMS Publishing). Data are presented as means and standard deviations. A P value < 0·05 was considered as statistically significant.
Results
Estimating the dietary vitamin C requirement of senescence marker protein-30 knockout mice
Because the RDA for VC is 100 mg/d in Japan( Reference Levine, Wang and Padayatty 6 ), we estimated that the daily dietary VC requirement of SMP30KO mice was 20 mg/kg per d based on normalisation using the body surface area. To evaluate whether this estimate was appropriate, SMP30KO mice were fed a purified diet containing 0·02 % VC (20 mg/kg per d) for 5 months and the plasma ascorbic acid level of SMP30KO mice was compared with that of age-matched C57BL/6 (WT) mice. The result revealed that the plasma ascorbic acid concentration of SMP30KO mice was almost the same as that of age-matched WT mice (6·6 (sd 1·7) and 6·8 (sd 0·8) mg/l, n 7 and n 5, respectively) after 5 months on a diet containing 0·02 % VC (20 mg/kg per d). This demonstrated that the daily dietary VC requirement of SMP30KO mice was 20 mg/kg per d.
Effects of high vitamin C intake on body weight, organ weights and plasma ascorbic acid level in senescence marker protein-30 knockout mice
To examine the influence of long-term high-dose VC intake on age-related changes in body weight, spleen and thymus weights, and plasma ascorbic acid level, twenty-one SMP30KO mice (mean body weight 21·4 (sd 0·4) g) were randomly divided into two groups and were fed a diet containing the recommended VC level (0·02 %, n 10) or a 10-fold higher level of VC (0·2 %, n 11) for 1 year. No serious adverse events were observed in both experimental groups throughout the study. Body weight and spleen weight showed no significant differences between the two groups, whereas thymus weight was significantly higher in the 0·2 % VC group than in the 0·02 % VC group after 1 year on the experimental diets. The plasma ascorbic acid level was also significantly higher in the 0·2 % VC group than in the 0·02 % VC group, which had a similar level to that of the age-matched WT group (Table 2).
Table 2 Effects of high dietary intake of vitamin C (VC) on body weight, thymus and spleen weights, and plasma ascorbic acid concentration in senescence marker protein-30 knockout mice (Mean values and standard deviations)
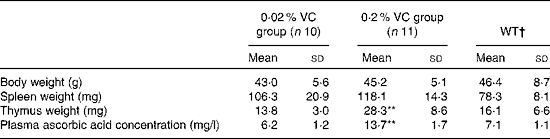
WT, wild type.
Mean value was significantly different from that of the 0·02 % VC group: **P< 0·01 (unpaired Student's t test).
† Data obtained from age-matched WT mice are shown as reference (n 5).
Effects of high vitamin C intake on peripheral blood cells, splenocytes and thymocytes
To investigate the effect of high VC intake on age-related changes in immune cells, we measured the total number and the populations of leucocytes, splenocytes and thymocytes after 1 year on the respective experimental diets. As shown in Table 3, the total number of leucocytes, lymphocytes, granulocytes and monocytes in the peripheral blood was significantly higher in the 0·2 % VC group than in the 0·02 % VC group. The total number of splenocytes and thymocytes was also markedly higher in the 0·2 % VC group than in the 0·02 % VC group. Immune cell numbers in the 0·02 % VC group were approximately the same as those in the WT group (Table 3).
Table 3 Effects of high dietary vitamin C (VC) intake on the number of peripheral blood cells, splenocytes and thymocytes in senescence marker protein-30 knockout mice (Mean values and standard deviations)
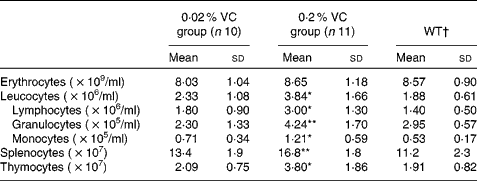
WT, wild type.
Mean value was significantly different from that of the 0·02 % VC group: *P< 0·05, **P< 0·01 (unpaired Student's t test).
† Data obtained from age-matched WT mice are shown as reference (n 5).
Effects of high vitamin C intake on the subpopulations of leucocytes, splenocytes and thymocytes
It is well known that thymic involution occurs with ageing, leading to a decrease in the number of naive T cells in the peripheral blood( Reference Gruver, Hudson and Sempowski 1 , Reference Elmore 25 , Reference den Braber, Mugwagwa and Vrisekoop 26 ). Therefore, we assessed T-cell populations after 1 year on the respective experimental diets. Among peripheral blood lymphocytes, the number of CD44lowCD45RBhigh cells in both CD4+ and CD8+ T-cell populations (naive CD4+ and CD8+ T cells, respectively) was significantly higher in the 0·2 % VC group than in the 0·02 % VC group, as were the number of CD44highCD45RBlow cells in both CD4+ and CD8+ T cells (memory CD4+ and CD8+ T cells, respectively) among splenocytes. Among thymocytes, although there was no significant difference in double-negative cells, the number of double-positive cells and CD4+ or CD8+ SP cells was significantly higher in the 0·2 % VC group than in the 0·02 % VC group (Table 4).
Table 4 Effects of high dietary vitamin C (VC) intake on the T-cell subpopulations of peripheral blood lymphocytes, thymocytes and splenocytes in senescence marker protein-30 knockout mice

WT, wild type; CD, cluster of differentiation; DN, double negative; DP, double positive; SP, single positive.
Mean value was significantly different from that of the 0·02 % VC group: *P< 0·05 (unpaired Student's t test).
† Data obtained from age-matched WT mice are shown as reference (n 5).
Discussion
In the present study, long-term high dietary intake of VC (at ten times the RDA) inhibited age-related thymic atrophy and maintained immune cells such as peripheral blood leucocytes, thymocytes and splenocytes in VC-deficient SMP30KO mice. In addition, the number of naive T cells in the peripheral blood and the memory T-cell populations of splenocytes was significantly higher in the 0·2 % VC group than in the 0·02 % VC group. Among thymocytes, the number of double-positive cells and CD4+ or CD8+ SP cell populations was also significantly larger in the 0·2 % VC group than in the 0·02 % VC group. These findings suggest that high dietary intake of VC over a long period contributes to sustaining immune cells, and could be effective in improving immune function in elderly people by preventing a decline in the number of immune cells.
SMP30KO mice cannot synthesise VC due to genetic disruption of the Rgn gene that encodes gluconolactonase, so this is a useful animal model for investigating the actions of VC. The daily dietary VC requirement of SMP30KO mice was estimated to be 20 mg/kg per d based on normalisation using the body surface area, and mice were fed a diet containing this VC level (20 mg/kg per d; 0·02 %) or a high VC level (200 mg/kg per d; 0·2 %). Plasma ascorbic acid concentration (Table 2) and the number of peripheral blood immune cells, splenocytes and thymocytes (Table 3) in SMP30KO mice fed a diet containing 0·02 % VC were similar to those in age-matched WT mice after 1 year on a conventional diet (CE-2). These observations suggested that SMP30KO mice from the 0·02 % VC group were similar to age-matched WT mice.
Age-related thymic atrophy is characterised by a progressive decrease in the size of the thymus and structural changes( Reference Gui, Mustachio and Su 27 ). Thymic atrophy has been demonstrated to be caused by a failure of the thymic microenvironment to support thymopoiesis and T-cell development( Reference Linton and Dorshkind 3 ). The extracellular matrix is an important component of the thymic microenvironment, which interacts with thymocytes to drive their trafficking inside the thymus. Lannes-Vieira et al. ( Reference Lannes-Vieira, Dardenne and Savino 28 ) studied the thymus in normal young mice and found that type I collagen is restricted to the interstitial spaces of the capsule and septae, whereas type IV collagen, fibronectin and laminin can be detected in the basement membrane, mostly in the inner cortex and outer medulla. Other researchers have reported that the size of both the cortex and medulla of the thymus decreases with age( Reference Pesic, Radojevic and Kosec 29 ). Because VC promotes the production of fibronectin, laminin and collagen by trabecular meshwork cells( Reference Zhou, Higginbotham and Yue 30 ), these components of the thymic extracellular matrix may be sustained in SMP30KO mice by long-term high dietary VC intake, and this may be one mechanism by which thymic atrophy was inhibited in the present study.
Thiobarbituric acid-reactive substances are a marker of oxidative stress that increase in the thymus with age, followed by the acceleration of thymic involution and the loss of thymocytes( Reference Kujoth, Hiona and Pugh 31 – Reference Sverko, Balog and Sobocanec 33 ). Melatonin is a strong antioxidant, and either long-term (8 months) or short-term (2 months) melatonin consumption prevents age-dependent thymic atrophy and attenuates apoptotic thymocyte death caused by free radicals in aged BALB/c and C57BL/6 mice( Reference Tian, Zhang and Dai 22 , Reference Provinciali, Di Stefano and Bulian 34 ). It is possible that the high dose of VC used in the present study had a strong antioxidant effect, inhibiting both age-dependent involution of the thymus and the resulting loss of thymocytes. There is a significant age-associated increase in apoptosis that affects the double-negative cell population of thymocytes, partly due to the elevated expression of the cell death gene caspase-3 in aged mice( Reference Andrew and Aspinall 35 , Reference Kapasi and Singhal 36 ). VC has been shown to inhibit thymocyte apoptosis by up-regulating the expression of the anti-apoptotic protein B-cell lymphoma-2( Reference Pavlovic, Pavlovic and Kamenov 37 – Reference Maellaro, Bello and Comporti 39 ). We found that the total number of thymocytes and the number of double-positive cells and CD4+ or CD8+ SP thymocyte subpopulations were markedly higher in the 0·2 % VC group than in the 0·02 % VC group after 1 year of dietary treatment, possibly reflecting the inhibitory effect of high-dose dietary VC intake on the apoptosis of thymocytes through the induction of B-cell lymphoma-2. In contrast, recent studies have shown that the SMP30 protein has a suppressive effect on apoptosis induced by various factors( Reference Yamaguchi 40 ), although it is unclear how much of the SMP30 protein is involved in inhibiting age-related thymic involution and the apoptosis of thymocytes. Because the thymus weight and plasma VC level of SMP30KO mice fed the diet containing 0·02 % VC were almost the same as those found in age-matched WT mice that had a sufficient amount of the SMP30 protein (Table 2), the inhibitory effect of SMP30 on thymic atrophy might be smaller than that of VC.
We also demonstrated that the number of peripheral blood lymphocytes, particularly naive CD4+ and CD8+ T cells, that have the ability to fight new infections, was significantly higher in the 0·2 % VC group than in the 0·02 % VC group (Table 4). An age-related deterioration of thymic production is thought to be one of the major factors leading to a decline in the number of naive T cells in the peripheral blood( Reference Gruver, Hudson and Sempowski 1 ). The long-term high dietary intake of VC may have ameliorated this decline of thymic cellular output, providing a stable supply of naive T cells in aged mice. We do not know why memory T cells were sustained at a high level among splenocytes in the 0·2 % VC group; however, this may have been related to the maintained thymic production of naive T cells.
The number of peripheral blood granulocytes or monocytes, which are recruited from the bone marrow to the periphery without passing through the thymus, was also higher in the 0·2 % VC group than in the 0·02 % VC group. Haematopoietic stem cells in the bone marrow generate multiple haematopoietic lineages through a series of intermediate progenitors. These progenitor cells include common lymphoid progenitors that can generate B cells, T cells or natural killer (NK) cells, and common myeloid progenitors that can generate erythrocytes, platelets, granulocytes or monocytes( Reference Akashi, Traver and Miyamoto 41 , Reference Kondo, Weissman and Akashi 42 ). Mesenchymal stem cells are a population of pluripotent cells within the bone marrow microenvironment, which participate in the regulation of the self-renewal and differentiation of haematopoietic stem cells( Reference Larghero, Vija and Lecourt 43 ). Several studies have indicated that VC can induce proliferation and differentiation of bone marrow-derived mesenchymal stem cells in a dose-dependent manner( Reference Choi, Seo and Yoon 44 ). Therefore, it seems that high dietary VC intake improves age-related dysfunction of the bone marrow microenvironment, thereby permitting the maintenance of a wide variety of immune cells, not only T cells that are produced in the bone marrow and matured within the thymus, but also granulocytes and monocytes that are released directly into the peripheral blood from the bone marrow. It is well established that ageing affects acquired or innate immune functions as well as the number of immune cells( Reference Srinivasan, Maestroni and Cardinali 45 ). To clarify the effect that VC has on various immune functions, further studies employing SMP30KO mice will be needed.
In conclusion, we demonstrated that high dietary intake of VC inhibited age-related thymic involution and maintained thymic output, resulting in stable peripheral immune cell counts in aged SMP30KO mice. The results from the present study suggest that long-term high dietary intake of VC may be effective in the maintenance of immune cells in elderly people.
Acknowledgements
The authors greatly appreciate Dr Norio Yamamoto for devising the HPLC method with electrochemical detection. The authors also thank Dr Koutarou Muroyama, Kengo Kawasaki, Tatsuya Ohara, Youhei Higashi and Yuuske Tanaka for their technical assistance.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The author's contributions are as follows: R. U., Y. H. and S. M. designed the study; R. U. conducted the study; R. U. and Y. H. analysed the data; Y. Y. and A. I. participated in the interpretation of the results; R. U. wrote the manuscript; S. M. had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no conflict of interest.









