Low-cost agro-industrial co-products are increasingly being used in pig diets, resulting in increased dietary fibre (DF) content. High-fibre diets are supplemented with lipids to meet the energy requirement for a nutritionally balanced diet for pigs. A common assumption is that growth performance in pigs fed nutritionally balanced high-fibre diets that are supplemented with added lipids will not decline. However, pigs fed fibrous diets based on cereal co-products plus supplementary fat reportedly had lower growth performance and nutrient utilisation, compared with those offered high-fibre diets that are composed of cereals or cereal co-products without added fats( Reference Bach Knudsen 1 – Reference Ravindran, Tancharoenrat and Zaefarian 5 ). This paradoxical relationship suggests that the understanding of the interaction between DF solubility and the degree of saturation of fatty acids (FA) in dietary lipids in pigs is far from complete.
The interactions between dietary fibre and lipid types may be hydrogen bonding or electrostatic as well as hydrophobic interactions( Reference Capuano 6 ). During these interactions, DF polysaccharides may non-covalently bind, adsorb or entrap other dietary components during transit along the gastrointestinal tract (GIT), and this capacity can also explain the changes in fermentation patterns as well as the FA flows in the GIT( Reference Capuano 6 ). Furthermore, agro-industrial co-products may contain TAG (neutral fats) and phospholipids embedded within their fibre matrices. The fibre-bound lipids may inevitably impose confounding effects that makes it difficult to understand the role played by added fats. Supplementary lipid sources include animal fats such as beef tallow that are rich in SFA or plant oils such as maize oil that are rich in unsaturated FA( Reference Ravindran, Tancharoenrat and Zaefarian 5 ). Unlike the cereal co-products that are rich in cellulose and have relatively minimal amounts of pectin, co-products from fruits, protein rich or oil-seed meals contain relatively high levels of pectin polysaccharides( Reference Bach Knudsen 1 , Reference Agyekum and Nyachoti 2 ). In this respect, the plant cell wall structure in co-products is made up of a network of cellulose fibrils and hemicellulose embedded in a network of pectins( Reference Bach Knudsen 1 , Reference Agyekum and Nyachoti 2 , Reference Capuano 6 ), however, the relative proportions of these DF constituents also vary in natural sources of fibre. The latter confers plasticity and controls porosity of the cell wall, whereas the former functions as a load-bearing structure( Reference Capuano 6 ). Thus, we suggested that the use of purified sources of DF such as cellulose and pectin that are lipid-free and well-characterised models for insoluble dietary fibre (IDF) and soluble dietary fibre (SDF), respectively, would open the way for investigating the interaction between DF and lipid types, by eliminating the confounding effects that may be imposed by the fibre matrice-bound lipids. SDF are highly fermentable and believed to influence the digestion process in the stomach and small intestines, whereas IDF primarily modulate processes in the caecum and colon where, due to their physical presence, they effectively increase faecal bulk, dilute hindgut contents and increase mouth to anus passage rate( Reference Bach Knudsen 1 , Reference Capuano 6 ). Thus, pectin and cellulose were regarded as soluble and insoluble fibre sources, respectively, and were used in this trial to create different conditions in different segments of the GIT. The addition of DF beyond optimum inclusion level may constrain feed intake or reduce digestibility of dietary components at a magnitude that depends on the source or the fermentability of the DF source or its ability to change digestion and alter different conditions in each segment of the GIT( Reference Bach Knudsen 1 , Reference Capuano 6 , Reference Ndou, Gous and Chimonyo 7 ). Taken together, the addition of DF-rich co-products may not only reduce feed cost but promote DF fermentation and modulate gastrointestinal health benefits and performance by modifying the gastrointestinal milieu, microbiota and nutrient absorption( Reference Capuano 6 – Reference Gutierrez, Kerr and Patience 11 ).
In ruminants, it is well established that high-fat diets can inhibit DF fermentation by gastrointestinal microbiota( Reference Brooks, Garner and Gehrke 12 ) or reduce microbiota population( Reference Mallett and Rowland 13 ) and diversity. The idiosyncrasy of the interaction between DF and lipid type on performance and gastrointestinal fermentation in pigs has been associated with microbial activity. Therefore, it can be postulated that the response of pigs to supplemented lipids in high-fibre diets is greatly influenced by the inclusion level of DF and/or its solubility. In pigs, soluble fibre is mostly fermented before reaching the colon but insoluble fibre is highly fermented after passing the caecum( Reference Jaworski and Stein 14 ). The diverse physicochemical properties of added lipids as well as their potential interactions with different types or sources of DF, minerals and microbiological activity may affect how fibre fermentation changes the conditions in different regions of the GIT and consequently the utilisation of added lipids in pigs. A common assumption is that SCFA (major products and indicators of fermentation) are rapidly absorbed and/or metabolised in the GIT. However, it is peculiar that the consumption of highly fermentable DF in pigs not only increased hindgut SCFA production and flows but also increased the amount of faecal fat and excretion of bile acids (BA)( Reference Graham, Hesselman and Åman 15 – Reference Ndou, Kiarie and Thandapilly 17 ). It is still a matter of debate on whether faecal fat could be attributable to endogenous losses from the gastrointestinal secretions, microbial FA production or sub-optimal absorption of added lipids predisposed by encapsulation of FA within the DF matrices or by deconjugation of BA( Reference Bach Knudsen and Hansen 16 – Reference Kil, Sauber and Jones 20 ). Increased faecal fat excretion because of fibre fermentation or poor fermentation of DF due to added lipids is obviously counter-productive of fibre nutrition efforts. There is need, therefore, to characterise and/or estimate the FA flows in each segment of the GIT as this may indicate FA that originates from malabsorption of added lipids, microbial activities or endogenous secretions. Understanding the role played by different sources of DF in creating different conditions in the GIT and subsequently modulating gastrointestinal fermentation characteristics and intestinal FA flows opens ways for formulation of high-fibre diets that optimise growth performance with minimum nutrient losses. Furthermore, this study could enhance the development of fundamental knowledge on how interaction of DF solubility and lipid type modulate pig performance and lipid nutrition. This is important to develop nutritional interventions to optimise dietary inclusion of high-fibre co-products as low-cost ingredients with desirable functional properties.
To the best of our knowledge, this study is the first to use ileal and caecal cannulated pigs to investigate the interactive effects of DF sources and degree of saturation of FA in supplementary lipids on digestibility of FA and FA flows in the ileum, caecum and the entire GIT. The hypothesis tested in this study was that dietary inclusion of either cellulose or pectin will create different conditions in the GIT, thereby modulating FA digestibility and gastrointestinal flow of FA, depending on the source of dietary lipids. Thus, the objective of the current study was to determine the interactive effects of DF type and source of dietary lipids on FA flows and the digestibility of dietary components in the ileum, caecum and the entire GIT using ileal and caecal cannulated pigs that were fed cellulose- or pectin-containing diets with either maize oil or beef tallow supplementation.
Methods
The experimental procedures and use of pigs in this trial were approved by the Animal Care Committee of the University of Guelph (protocol no. 1938). Pigs were cared for according to the guidelines of the Canadian Council on Animal Care( 21 ).
Pigs and housing
In brief, a total of eight Yorkshire barrows (25·3 (sd 1·67) kg mean body weight (BW)) were housed individually in pens (1·2 × 1·8 m). The temperature in the room was maintained at 22 ± 2·3°C with a 14 h light and 10 h dark cycle. After a 7 d adaptation period to experimental room, pigs were surgically equipped with two simple T-cannulas. Before surgeries, pigs were injected with a Primix (carprofen (2 mg/kg BW; 50 mg/ml), Excenel (0·06 ml/kg BW; 100 mg/ml; Excenel® RTU EZ, ceftiofur hydrochloride, sterile suspension; Zoetis Inc.), bupivacaine (2 ml/25 kg BW) and buprenorphine (0·01 mg/kg BW; 0·3 mg/ml) to prevent pain and infections. The first cannula was inserted at the terminal ileum( Reference Nyachoti, McNeilage-Van de Wiele and De Lange 22 ) and the second cannula was placed in the caecum approximately 5 cm proximal to the caecocolic junction. The ileal and caecal cannulated pig model was used in this study because it opens ways for collecting ileal and caecal digesta separately to assess fermentation of DF and FA flows in the uppergut, caecum and colorectal tract. During and after the surgery and recovery period, welfare-related assessments such as body temperature, feeding and general behaviour were conducted and recorded for each pigs. After surgery, pigs were allowed a recovery period of 10 d. During the recovery period, the cannulated pigs were given Metacam (0·4 mg/kg; 15 mg/ml; Metacam® for Swine; Boehringer Ingelheim) oral suspension for swine to control inflammation and pain. The areas around the cannulas were cleaned twice a day with an antiseptic soap and a zinc oxide cream was applied to prevent pain and infection. Each pen was equiped with welfare-enrichment toys and chains to improve the welfare conditions of the pigs. All pigs were healthy and finished their daily feed allowances.
Experimental diets, design and management procedures
The pigs were fed high-fibre diets that were formulated to contain 150 g/kg of either cellulose (Cellulose powder BH65 FCC; Cambrian Chemicals Inc.) or pectin (Citrus pectin, GENU® pectin type VIS; CP Kelco) and either 62 g/kg of maize oil or 63·7 g/kg of beef tallow. In each diet, either pectin or cellulose was the only source of DF. Either maize oil or beef tallow was added in each diet, as the only source of dietary FA. Thus, giving a 2 × 2 factorial arrangement of treatments. The diets were formulated to meet nutrient requirements of growing pigs, as prescribed by the National Research Council (NRC)( 23 ) (Table 1). Titanium dioxide was included (3·0 g/kg) as an indigestible marker in all diets.
Table 1 Ingredients and analysed compositions (g/kg) of the experimental diets
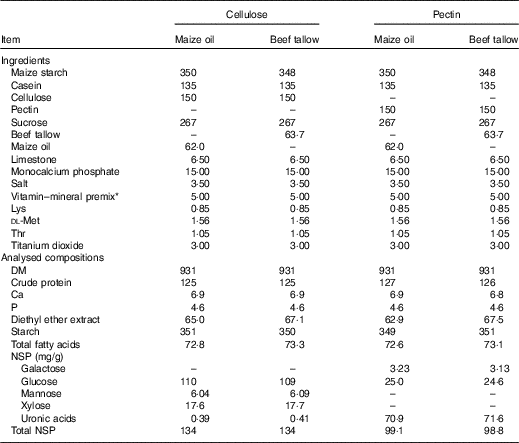
* Vitamin–mineral premix provided the following nutrients (per kg of air-dry diet): vitamin A, 0·60 mg; vitamin D3, 0·01 mg; vitamin E, 40 mg; vitamin K, 2 mg; vitamin B1, 1·5 mg; vitamin B2, 7 mg; vitamin B6, 2·5 mg; vitamin B12, 25 µg; calcium pantothenate, 14 mg; folic acid, 1 mg; niacin, 21 mg and biotin, 70 µg. Minerals: Cu, 10 mg (as copper sulphate); iodine, 0·4 mg (as potassium iodine); Fe, 120 mg (as ferrous sulphate); Mn, 10 mg (as manganous oxide); Se, 0·3 mg (as sodium selenite) and Zn, 110 mg (as zinc oxide).
A total of eight pigs were used in two blocks, four pigs per block. In each block, pigs were randomly assigned to the diets in a Youden square design with four treatments (diets), three columns (experimental periods) and four rows (pigs), to give six replicates per treatment (n 6). Pigs were weighed at the beginning of each experimental period and offered a daily feed ration equivalent to supply 2·8 times the estimated requirement for maintenance energy (i.e. 197 kcal (824 kJ) metabolisable energy/BW0·6 kg; NRC( 23 )). The daily feed ration was offered in two equal meals at 08.00 and 20.00 hours. Pigs were offered feed that was mixed with water (1:2, or 500 g of feed/l (w/v)) to improve palatability. All pigs finished their daily feed allowance throughout the experiment.
Each experimental period lasted 15 d: 9 d for acclimatisation to the experimental diets, followed by 2 d for faecal, 2 d for caecal contents and 2 d for ileal digesta collection. Using the grab sample technique, fresh faecal samples were collected hourly and placed into plastic bags and immediately frozen at –20°C. Ileal and caecal digesta were collected from each pig by attaching a sterile 500 ml plastic bag to the cannula barrel. Digesta and faecal samples were collected over a 12 h period between 08.00 and 20.00 hours. However, throughout the sample collection period, plastic bags were filled with 10 ml of 10 % formic acid. The bags were changed every 30 min unless filled with digesta. After collection, digesta and faecal samples were thawed to semi-solid state, pooled within pig and collection period, freeze-dried and stored at –80°C until analysed.
Laboratory analysis
The diets, faeces, ileal and caecal digesta were analysed (Table 2) in duplicates for DM, starch and ash following standard procedures( 24 ), and titanium as described by Lomer et al. ( Reference Lomer, Thompson and Commisso 25 ). Total starch was determined using a test kit (Megazyme International Ireland). The diets were also analysed for crude protein, gross energy, diethyl ether extract, Ca, P, NSP contents as described by Ndou et al. ( Reference Ndou, Kiarie and Thandapilly 17 ). Lipids were extracted from diets, digesta and faeces using a 2:1 chloroform–methanol mixture( Reference Folch, Less and Sloane-Stanley 26 ) and methylated to produce FA methyl esters( Reference Metcalfe and Schmitz 27 ). All FA from C8 : 0 to C24 : 1 were analysed using GC but only specific FA present in high concentration were reported.
Table 2 Fatty acid profile (g/100 g of total fatty acids) of experimental diets
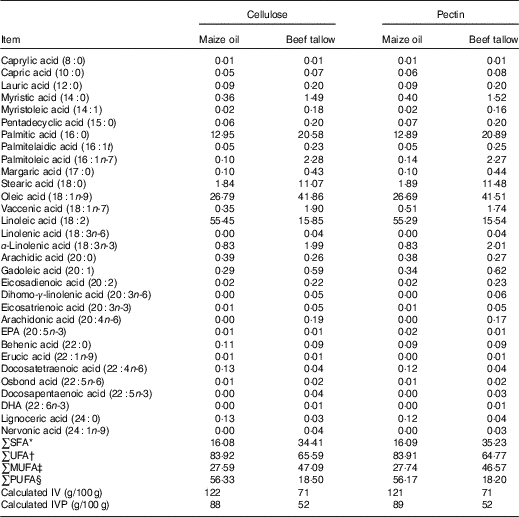
IV iodine value; IVP, iodine value of the product.
* ∑SFA = total SFA.
† ∑UFA = total unsaturated fatty acids.
‡ ∑MUFA = total MUFA.
§ ∑PUFA = total PUFA.
Calculations and statistical analyses
Iodine values of diets
The iodine values (IV) and iodine value of the product (IVP) of the experimental diets were calculated as follows:
IV = ((C16 : 1) × 0·95)+((C18 : 1) × 0·86)+((C18 : 2) × 1·732)+((C18 : 3) × 2·616)+((C20 : 1) × 0·785)+((C22 : 1) × 0·723), in which the brackets indicate concentration (percentage) of FA (adopted from American Oil Chemists’ Society( 28 )).
IVP = (IV of dietary lipids) × (% dietary lipid) × 0·10 (adopted from Madsen et al. ( Reference Madsen, Jakobsen and Mortensen 29 )).
Digestibility and flows
The apparent ileal digestibility (AID), apparent caecal digestibility (ACD) and apparent total tract digestibility (ATTD) digestibilities were calculated as follows:
where NutrientF/C/I are the contents of dietary components (g/kg DM) in the faeces (F), caecal (C) or ileal (I) digesta, respectively; NutrientD is the content of each nutrient (g/kg DM) in the diet; T D is the titanium dioxide (g/kg DM) in the diet and T F/C/I are the concentrations of titanium dioxide (g/kg DM) in faeces, caecal or ileal digesta, respectively.
The flow of FA in the stomach and small intestines, caecum or colon was calculated using the following equation:
where Flownutrient is the flow of dietary components; NutrientI/C/F is the concentration of each dietary component in faeces (F), ileal (I) or caecal (C) digesta, respectively.
Statistical analyses
Statistical analyses were performed using SAS (version 9.4, 2009; SAS Institute Inc.)( 30 ). A power test analysis estimated according to Bowley( Reference Bowley 31 ) and based on data obtained from a recent study( Reference Jaworski and Stein 14 ) on digestibility and flows of dietary components in ileal- and caecal-cannulated pigs was performed to determine the number of replicates per treatment needed for the present experiment. On the basis of the same effect size, the power test analysis indicated that statistical power of more than 90 % for a sample size of six and α = 0·05 could be expected, enabling adequate power to reject the null hypothesis when it is false (P = 1–β). In this regard, our proposed sample size of six replicates per treatment may provide more power when considering multiple comparisons.
The data were analysed as a completely randomised design with 2 × 2 factorial treatment arrangements using the GLIMMIX procedure. The models accounted for the main effects of DF and lipid types and associated two-way interactions as fixed factors. The pig, block and period were included as random effects. The pig (nested within period) was considered as the experimental unit for all analyses. A total of three contrast statements were also tested to compare the effect of DF source (cellulose v. pectin), lipid type (maize oil v. beef tallow) and the interactions of DF and lipid types. Comparisons of means for the interactions between DF and lipid types were performed using the Tukey–Kramer honestly significance difference test. A P-value of ≤0·05 was used to determine significance, and trends declared for P-values between 0·05 and 0·10 were discussed.
Results
All pigs consumed their assigned diets without refusals or spillages and faecal, caecal and ileal digesta samples were successfully collected from all pigs.
Apparent ileal digestibility and fermentability
There were interactions (P <0·05, Table 3) between DF and lipid types on AID of DM, SFA, MUFA, PUFA and total fatty acids (TFA). In this regard, the addition of beef tallow increased (P <0·05) the AID of DM in the pectin-containing diet but did not induce effects in the diet with cellulose. Dietary supplementation with beef tallow increased (P <0·05) the AID of SFA and PUFA in both pectin- and cellulose-containing diets. The addition of beef tallow reduced the AID of MUFA (P = 0·001) in the cellulose-containing diet but increased in the pectin-containing diet. Dietary supplementation with beef tallow decreased (P <0·05) the AID of TFA in the pectin-containing diet but did not have effects in the cellulose-containing diet. There were no significant interactive effects of DF and lipid types on AID of N and starch, but the main effects of DF increased the AID of starch in cellulose-containing diets (P <0·05) compared with pectin-containing diets.
Table 3 Apparent ileal, caecal and total tract digestibility (%) of dietary components by growing pigs fed experimental dietsFootnote * (Mean values with their standard errors)
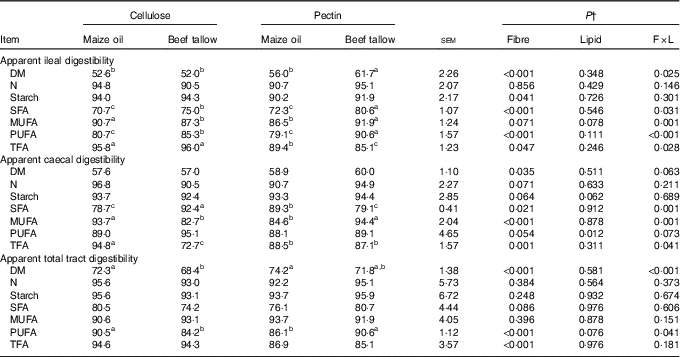
TFA, total fatty acids.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Mean values with their pooled standard errors (n 6 per treatment).
† Contrast statements: fibre=cellulose v. pectin; lipid= maize oil v. beef tallow; F×L=interaction of dietary fibre and lipid type.
Apparent caecal digestibility and fermentability
There was an interaction (P <0·05; Table 3) between DF and lipid types on the ACD of SFA, MUFA and TFA. The addition of beef tallow increased the ACD of SFA in cellulose-containing diets but decreased in diets containing pectin (P = 0·001). Supplementary beef tallow decreased the ACD of MUFA in the diet containing cellulose but increased in the pectin-containing diet (P <0·05). Dietary addition of beef tallow decreased (P <0·05) the ACD of TFA in cellulose-containing diets but did not induce effects in pectin-containing diets. There were no interactions (P >0·05) between DF and lipid types on ACD of DM, N, starch and PUFA, however, the main effects of DF were such that diets containing pectin had greater (P <0·05) ACD of DM compared to diets containing cellulose. The main effects of dietary lipid type increased the ACD of PUFA in diets supplemented with beef tallow compared with diets containing supplementary maize oil (P <0·05).
Apparent total tract digestibility and fermentability
An interaction (P <0·05) of DF and lipid type was observed on ATTD of DM and PUFA but not (P >0·05) on ATTD of N, starch, SFA, MUFA and TFA (Table 3). In this regard, the addition of beef tallow decreased (P <0·001) the ATTD of DM in the cellulose-containing diet but did not have effects in pectin-containing diets. Dietary supplementation with beef tallow decreased the ATTD of PUFA (P <0·01) in cellulose-containing diets but increased in diets containing pectin. The main effects of DF type decreased the (P <0·001) ATTD of TFA in pectin-containing diets compared with cellulose-containing diets.
Fatty acid flows in the ileum
There were interactions (P <0·05; Table 4) between DF and lipid types on the ileal flow of 8 : 0, 16 : 1t, 16 : 1n-7, 18 : 0, 18 : 1n-9, 18 : 1n-7, 18 : 2, 18 : 3n-6, 20 : 0, 20 : 2 and 20 : 3n-3. Dietary supplementation with beef tallow increased the ileal flow of 8 : 0 and 18 : 2 in cellulose-containing diets but decreased in diets containing pectin (P <0·05). Dietary supplementation with beef tallow increased (P <0·01) the flow of ileal 16 : 1n-7 in pectin-containing diets but did have effects in cellulose-containing diets. Dietary inclusion of beef tallow decreased (P = 0·001) the ileal flow of 18 : 0 in diets containing cellulose and pectin. The flow of 18 : 1n-9, 18 : 1n-7, 18 : 3n-6 and 20 : 2 in the ileum increased (P <0·05) with supplementation of beef tallow in both cellulose- and pectin-containing diets. The supplementation with beef tallow decreased the flow of 20 : 0 in the ileum in pectin-containing diets but did not have effects in the diet containing cellulose (P <0·05). The addition of beef tallow increased (P <0·05) the flow of 20 : 3n-3 in ileum in cellulose-containing diets but did not induce effects in pectin-containing diets.
Table 4 Ileal fatty acid flow in pigs fed experimental diets (mg/kg DM intake)Footnote * (Mean values with their standard errors)
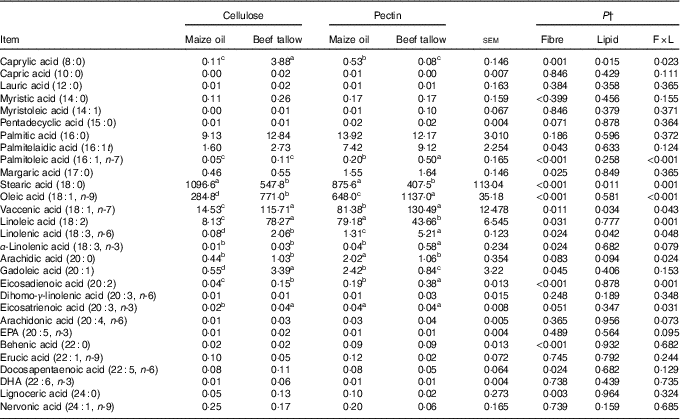
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P<0.05).
* Mean values with their pooled standard errors (n 6 per treatment).
† Contrast statements: fibre=cellulose v. pectin; lipid= maize oil v. beef tallow; F×L=interaction of dietary fibre and lipid type.
There were no (P >0·10) interactive effects of DF and lipids types in the ileal flows of 10 : 0, 12 : 0, 14 : 0, 14 : 1, 15 : 0, 16 : 0, 16 : 1t, 18 : 3n-3, 20 : 1, 20 : 3n-6, 20 : 4n-6, 20 : 5n-3, 22 : 0, 22 : 1n-9, 22 : 5n-6, 22 : 6n-3, 24 : 0 and 24 : 1n-9. The main effects of DF increased (P <0·05) the ileal flow of 16 : 1t and 17 : 0 in pectin-containing diets compared with cellulose-containing diets.
Fatty acid flows in the caecum
In the caecum, an interaction (P <0·01; Table 5) of DF and lipid type was observed on the flows of 8 : 0, 10 : 0, 14 : 0, 14 : 1, 15 : 0, 16 : 0, 16 : 1t, 16 : 1n-7, 17 : 0, 18 : 0, 18 : 1n-9, 18 : 1n-7, 18 : 2, 18 : 3n-6, 18 : 3n-3, 20 : 0, 20 : 1, 20 : 2, 20 : 4n-6, 22 : 0, 22 : 1n-9, 22 : 5n-3 and 24 : 1n-9. In this case, supplementary beef tallow increased (P <0·05) the flows of 8 : 0, 10 : 0, 14 : 0, 14 : 1, 15 : 0, 16 : 0, 16 : 1t, 16 : 1n-7, 17 : 0, 18 : 1n-7, 18 : 1n-9, 18 : 3n-3, 18 : 3n-6, 20 : 1, 20 : 2, 22 : 0, 22 : 1n-9 and 22 : 5n-3 in the caecum in diets containing pectin but did not have effects in cellulose-containing diets. The addition of beef tallow decreased (P <0·05) the caecal flow of 18 : 0, 18 : 2, 20 : 0 and 20 : 4n-6 in pectin-containing diets but did not have effects in cellulose-containing diets. The dietary supplementation with beef tallow decreased the flow of caecal 24 : 1n-9 in both cellulose- and pectin-containing diets. There were no significant interactive effects of DF and lipid types on the caecal flows of 12 : 0, 20 : 3n-6, 20 : 3n-3, 20 : 5n-3, 22 : 4n-6, 22 : 6n-3 and 24 : 0.
Table 5 Caecal fatty acid flow in pigs fed the experimental diets (mg/kg DM intake)Footnote * (Mean values with their standard errors)
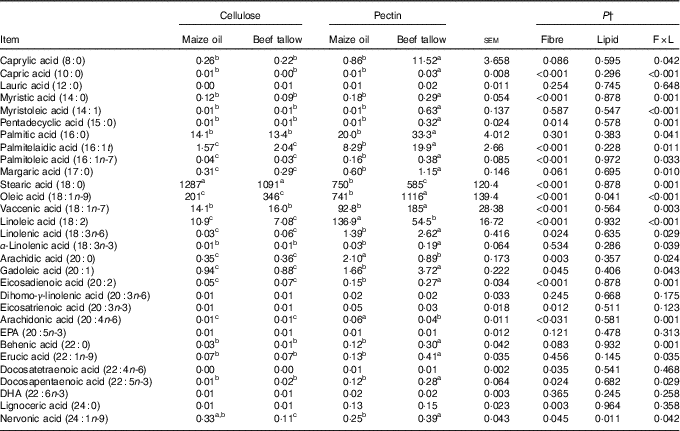
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Mean values with their pooled standard errors (n 6 per treatment).
† Contrast statements: fibre=cellulose v. pectin; lipid= maize oil v. beef tallow; F×L=interaction of dietary fibre and lipid type.
Flow of fatty acids in faeces
There was an interaction (P <0·05; Table 6) between DF and lipid type on faecal flow of 8 : 0, 12 : 0, 14 : 1, 16 : 1t, 18 : 2, 20 : 0, 20 : 3n-6, 20 : 5n-3, 22 : 1n-9, 22 : 5n-3 and 24 : 0. The addition of beef tallow decreased (P <0·05) the flow of faecal 8 : 0, 14 : 1, 16 : 1t, 18 : 2, 20 : 0, 20 : 3n-6 and 20 : 5n-3 in the diet containing pectin but did not induce effects in the cellulose-containing diet. Dietary supplementation with beef tallow decreased faecal flows of 12 : 0, 22 : 5n-3 and 24 : 0 in cellulose- and pectin-containing diets. The addition of beef tallow decreased (P >0·05) the caecal flow of 22 : 1n-9 in the diet containing cellulose but did not have effects in the pectin-containing diet. There were no (P >0·05) interactive effects between DF and lipid types on the faecal flow of 10 : 0, 14 : 0, 15 : 0, 16 : 0, 16 : 1n-7, 17 : 0, 18 : 0, 18 : 1n-9, 18 : 1n-7, 18 : 2, 18 : 3n-6, 18 : 3n-3, 20 : 1, 20 : 2, 20 : 3n-3, 20 : 4n-6, 22 : 0, 22 : 4n-3 and 24 : 1n-9 (P >0·05). The main effects of DF type increased (P >0·05) the faecal flow of 10 : 0, 20 : 3n-3, 20 : 4n-6 and 22 : 0 in diets containing pectin compared with cellulose-containing diets. The main effects of lipid type decreased (P >0·05) the flow of faecal 15 : 0 in the diet containing beef tallow compared with the diet containing maize oil. The main effects of DF type decreased (P >0·05) the flow of faecal 18 : 0 in diets containing pectin compared with the cellulose-containing diets.
Table 6 Faecal fatty acid flow in pigs fed the experimental diets (mg/kg DM intake)Footnote * (Mean values with their standard errors)
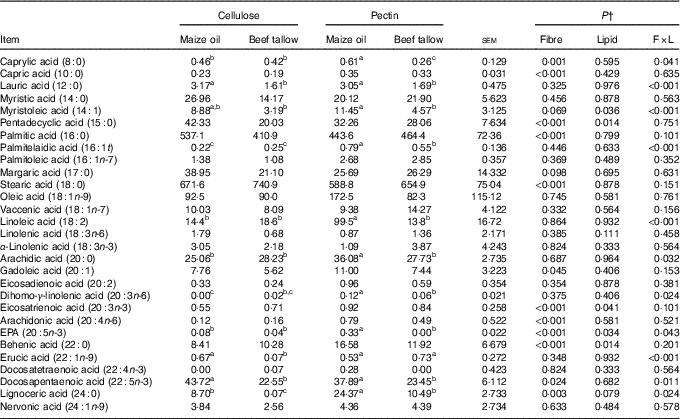
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Mean values with their pooled standard errors (n 6 per treatment).
† Contrast statements: fibre=cellulose v. pectin; lipid= maize oil v. beef tallow; F×L=interaction of dietary fibre and lipid type.
Discussion
The soluble, insoluble and total DF contents of the diets were not determined in this study, however, as expected, the diets containing pectin and those containing cellulose differed substantially in the content of NSP. Cellulose is an insoluble fibre made up of straight chains of glucose units that are generally linked by β-(1→4) bonds( Reference Bach Knudsen 1 , Reference Agyekum and Nyachoti 2 , Reference Capuano 6 ). Conversely, pectin is a branched polymer of galacturonic acids that are linked by α-(1→4) glycosidic linkages and is classified as a soluble fibre( Reference Bach Knudsen 1 , Reference Agyekum and Nyachoti 2 , Reference Capuano 6 ). Therefore, due to the differences in their well-known solubility properties and monomeric compositions, cellulose and pectin polysacharides were incorporated in diets used in this trial to create different conditions in different segments of the GIT. Moreover, the slightly higher fat content in beef tallow diets than in maize oil diets is attributable to high inclusion in diet formulation to achieve isoenergetic values across all diets as it is well known that beef tallow has a lower energy value than maize oil. The TFA contents were similar among all diets but the SFA, unsaturated FA, MUFA and PUFA profiles were different between beef tallow- and maize oil-containing diets.
The observation that addition of maize oil in pectin diets depressed digestibility of DM in the ileum was unexpected and contradicts our observation that dietary supplementation with beef tallow depressed digestibility of DM in cellulose-containing diets in the entire GIT. These discrepancies could be attributed to the variation in the endogenous enzymatic activity and differences in microbiota compositions and activity induced by the uniqueness in the behaviour of the DF and its influence on physicochemical properties of digesta in pigs fed different fibre sources( Reference Capuano 6 , Reference Ndou, Gous and Chimonyo 7 , Reference Ndou, Tun and Kiarie 32 ). Furthermore, the presence of charged regions within the branched galacturonic acid residues in pectin could have created more surface area for the non-convalent interactions with the absorbant to be formed for FA in beef tallow compared to cellulose straight chains that are less charged and closely packed together by the hydrogen bonds between the strands( Reference Capuano 6 ). Therefore, our observations further suggest that absorption of nutrients differs depending on the physicochemical property of the fibre source, the segment of the gut as well as the level of saturation of FA in added dietary lipids( Reference Capuano 6 , Reference Ndou, Gous and Chimonyo 7 , Reference Mallett and Rowland 13 , Reference Bach Knudsen and Hansen 16 ). The observation that AID of DM was greater and ACD of DM tended to be greater in pectin diets compared with cellulose diets concurs with the findings of Drochner et al. ( Reference Drochner, Kerler and Zacharias 33 ). Drochner et al. ( Reference Drochner, Kerler and Zacharias 33 ) predicted that the extent of depression of organic matter digestibility when 5% pectin is included in a diet will not be as high as with cellulose. Our data show that ileal and caecal digestibility of DM in pectin diets were greater compared with cellulose diets, which could be ascribed to the uniqueness of the constituent monomers between the two polysaccharides. Unlike cellulose which is made up of sugars joined together in straight chains to give it a hydrophobic and crystalline structure that is only modestly accessible to microbial degradation, pectins are branched polymers of galacturonic acids that are soluble and accessible to microbial fermentation( Reference Bach Knudsen and Hansen 34 , Reference Wilfart, Montagne and Simmins 35 ). Therefore, the branched chains in pectin offers a greater surface area for the interaction between the fibre and the FA in beef tallow compared cellulose polymers, resulting in inconsistencies in digestibility of not only DM but also dietary lipids depending on the degree of saturation of the constituent FA( Reference Mateos, Sell and Eastwood 36 ). Supporting this phenomenon is the notion that depending on their degree of saturation of the FA, lipids may depress microbial fibrolytic activity by forming a film of adsorbate on the surface of the fibre matrix, resulting in slow caption of amino acids and production of ATP needed to support bacterial fermentation( Reference Galbraith and Miller 37 ).
DF can inhibit the catalytic activity of digestive enzymes (including lipases) by interacting directly with the enzymes at molecular level through electrostatic, hydrogen bonding or hydrophobic interactions that alter the enzyme conformation( Reference Capuano 6 ). There was evidence for the interaction effect of DF and lipid types on AID of SFA, MUFA, PUFA and TFA, ACD of SFA, MUFA and TFA and ATTD of PUFA. The apparent digestibility of TFA at the end of the ileum and over the entire GIT was lower for pectin-containing diets than for diets containing cellulose. Although we did not quantify the fibre components in each diet, this is likely a result of greater concentration of soluble fibre fractions in digesta of pigs fed diets containing pectin compared to those fed diets containing cellulose. Drochner et al. ( Reference Drochner, Kerler and Zacharias 33 ) also observed that pectin depressed fat digestibility by binding BA and reducing their capacity to emulsify fats. Alternatively, pectin can inhibit pancreatic lipase by forming a complex with the enzyme thereby disrupting binding between the lipid droplets and enzyme active site or altering protonation of lipase active site by carboxylic acids residues of pectin( Reference Capuano 6 , Reference Isaksson, Lundquist and Ihse 38 , Reference Kumar and Chauhan 39 ). Moreover, the addition of beef tallow further depressed digestibility of TFA in pectin diets compared with cellulose diets, and this could be ascribed to high contents of 16 : 0 and 18 : 0 that contribute approximately 35% SFA in beef tallow. The AID of SFA decreases with increasing chain lengths( Reference Cera, Mahan and Reinhart 40 , Reference Cera, Mahan and Reinhart 41 ). Supplementation with beef tallow increased the ileal digestibility of SFA, but the increment was pronounced more in pectin diets compared with cellulose diets. Because a greater proportion of pectin disappears in the small intestines, implying that there is less interaction between DF fractions from pectin and SFA, yet in cellulose diets more IDF fractions from cellulose reach the distal regions of the intestines.
Previous reports by Pomerenke( Reference Pomerenke 42 ) and Davis et al. ( Reference Davis, Urriola and Shurson 43 ) reported that addition of 30 % IDF from dried distiller’s grains with solubles (DDGS) reduced digestibility of 16 : 0 and total SFA in comparison with addition of 0 or 5 % fibre from DDGS, which was likely due to an interaction between DF and lipid sources. Kim et al. ( Reference Kim, Kil and Stein 44 ) also proposed that fibre matrix surround the TAG and consequently impede the digestion process by preventing lipase from assessing the lipid. Therefore, these observations strongly support the notion that absorption of SFA are reduced when tallow is added to a diet that is rich in insoluble fibre compared with the one supplemented with soluble fibre. The same phenomenon was also noticed with AID of PUFA whereby the addition of beef tallow in pectin diets increased PUFA digestibility but supplementation with cellulose depressed digestibility. Furthermore, our findings are also in agreement with previous reports suggesting that digestibility of SFA, and 16 : 0 in particular, is greater in beef tallow-containing diets compared with other vegetable-based lipid sources such as rapeseed, sunflower and flaxseed oils( Reference Ozimek, Sauer and Kozikowski 45 – Reference Mitchaothai, Everts and Yuangklang 47 ). Another plausible explanation for the decrease in the digestibility of SFA in diets supplemented with maize oil could be due to an increase in the ileal flow of 18 : 0 as a result of its formation from biohydrogenation processes of PUFA and specifically 18 : 2 which increased in the GIT( Reference Martínez-Ramírez, Kramer and de Lange 48 ). In the present study, the dietary level of PUFA was high in maize oil-supplemented diets, however, biohydrogenation rates of PUFA are more pronounced in the hindgut than in the distal ileum( Reference Duran-Montgé, Lizardo and Torrallardona 46 , Reference Jørgensen, Gabert and Hedemann 49 ). In the present study, it is well established that MUFA represents over 50 % of the total lipids in pork fat deposits( Reference Xu, Baidoo and Johnston 50 ) and represents almost a third of TFA in maize oil diets and approximately one-half of the TFA in beef tallow-containing diets. The observation that SFA were numerically less digestible compared with MUFA and PUFA can be ascribed to the postulation that due to their lipid structure, SFA has a lower ability than MUFA or PUFA to form micelles and cross the water layer( Reference Jørgensen, Jakobsen and Eggum 51 – Reference Powles, Wiseman and Cole 53 ).
Apart from other dietary factors, the digestibility of lipids in the hindgut is influenced by several factors including endogenous secretion from mucosal cells, sloughing of epithelial cells or de novo synthesis by gastrointestinal microbiota( Reference González-Muñoz, Bastida and Sanchez-Muniz 54 ). It is interesting to note that regardless of the lipid or fibre source, the ACD of lipids was lower than the AID of lipids in all diets. The lower digestibility of ACD of lipids can be attributed to the effects of DF that initiated inefficient digestibility and sub-optimal absorption of dietary fat due to deconjugation of BA by increased bacterial activity in the intestinal lumen( Reference Ndou, Kiarie and Thandapilly 17 ). These processes reduce the solubility and emulsifying capacity of BA, and consequently they are bound to bacterial cells and DF, thereby increasing their excretion as reported in our previous study( Reference Ndou, Kiarie and Thandapilly 17 , Reference Ndou, Kiarie and Ames 55 ). Supporting these notions is the observed intestinal flow of FA that were virtually absent in the diets but increased in different sections of the GIT. For example, the flow of 8 : 0, 17 : 0, 18 : 3n-6, 20 : 3n-3, 18 : 1n-7, 20 : 4n-6 and 24 : 1, n-9 increased in the terminal ileum. The increases in n-3 and n-6 FA flow indicate a likelihood of activity of the Δ6- and Δ5-desaturases and chain-elongases in the upper sections of the GIT of the pig( Reference Martínez-Ramírez, Kramer and de Lange 48 ). More work is needed to explain the role played by gastrointestinal microbiota in elongating or desaturating FA such as 18 : 2n-6 and 18 : 3n-3 and the extent by which microbial fermentation contributes to an increase in the presence of unsaturated FA in the digesta of pigs. There was an increase in the caecal flow of 18 : 3n-6, 20 : 3n-3, 20 : 4n-6 and 22 : 5n-6 more in pigs that were fed pectin-containing diets than in those fed cellulose-containing diets, suggesting that microbiota played a central role in the production of these long-chain FA. Thus, the increase in flow of these FA could also be attributed to the action of microbiota such as Butyrivibrio fibrisolvens through biosynthesis or other unknown bacteria that can facilitate the biodehydrogenation processes( Reference Martínez-Ramírez, Kramer and de Lange 48 ). Furthermore, the finding that the flows of 18 : 1 decreased along the GIT occurred at the same time when 18 : 2 increased supports a fact that is well established in ruminants that feeding high-fibre diets stimulated the synthesis of the former through Δ9 desaturation from 18 : 1 precursor( Reference Bauman and Griinari 56 ). However, the increase in digestibility of lipids in diets containing cellulose and maize oil can be ascribed to the decrease in the flow of FA in the colon. Moreover, the lower ATTD of lipids in pectin-containing diets can be partly attributed to the content of mucilage in pectin or microbial production of FA following the conversion of SCFA into elongated forms of FA or the contribution of endogenous losses of FA from the host to the intestinal flow of FA. Nevertheless, even though long-chain FA cannot be absorbed in the hindgut, their production is obviously irrelevant to the NEFA pool in the porcine blood but could indirectly affect lipid metabolism by influencing microbial activities that play a central role in SCFA production.
In general, the AID of DM was lower than the ATTD values, whereas the values for ACF of DM were intermediate. The ileal and total tract digestibility values of DM in cellulose diets in the current study were lower than that reported by Hooda et al. ( Reference Hooda, Metzler-Zebeli and Vasanthan 57 ) with regard to ileal cannulated pigs fed 52·0 g/kg of cellulose in the diet. The inconsistencies in the digestibility values in this study and the previous report( Reference Hooda, Metzler-Zebeli and Vasanthan 57 ) can be attributed to the differences in the source and dietary inclusion levels of cellulose used in the two experiments. Although the marginal differences between AID and ACD of DM were more pronounced in the cellulose diets compared with pectin diets, they are in agreement with the previous notions postulated by Jaworski & Stein( Reference Jaworski and Stein 14 ) that a significant part of the DF fermentation occurs in the caecum. Furthermore, these marginal differences suggest that since microbial degradation of pectin fibres commences in the upper gastrointestinal segments( Reference Bach Knudsen, Lærke and Jørgensen 58 , Reference Montoya, Rutherfurd and Moughan 59 ), the fermentability of soluble fibre fractions of such pectin occurs at a lower extend in the caecum or colon compared to insoluble fibre components including cellulose. More work is required to determine the interactive effects of DF and lipid types on fermentability of DF fractions in different gut segments in growing pigs fed different sources of natural fibres and supplementary lipids. Although we cannulated in the caecum 5 cm proximal to the caecocolic junction, Jaworski & Stein( Reference Jaworski and Stein 14 ) predicted fermentability of DF in the caecum using cannulas that were inserted in the colon 20 cm distal to the caecum. In accordance with our findings, these authors also indicated that the fermentability of DF fractions varies along the GIT. Cannulation inside the caecum could have imposed some limitations to the caecal digestibility values especially with regard to the potential backflow that may arise due to the uncontrolled peristaltic movements of the gastrointestinal muscles. Taken together, our data suggest that the source of lipids supplemented in high-fibre diets also influence the extent of DM digestibility, also depending on the source of the DF.
Although this study had some limitations, its results also have implications that could be of interests not only in swine nutrition but also in human nutrition. In this regard, the study opens the ways to understanding the role played by interactive effects of DF sources and the degree of FA saturation in dietary lipids, and their ability to create different conditions in the gastrointestinal milieu. One limitation was that to achieve isoenergetic and iso-nutritious diets in feed formulation, 150 g/kg of either pectin or cellulose was included in each diet, resulting in a confounded higher content of NSP (13 %) in the cellulose-containing diets compared to the lower content of NSP (9·9 %) in pectin-containing diets. This implies that pigs fed diets with cellulose consumed significantly more DF compared with pigs fed diets with pectin. Another limitation was that there is a general increase in the flow of FA from one segment to the other, but it is still difficult to identify the actual source of lipids excreted in the terminal ileum, caecum or faeces. This implies that the use of isotope-labelled FA would open way for future studies to pinpoint whether FA in different gastrointestinal segments originate from the diet, endogenous secretions or microbial activities following deconjugation of BA and/or microbial bioconversion of dietary FA or endogenous lipids into microbial FA. Considering that human healthcare practitioners strongly recommend that patients increase DF in their rations and beef tallow tended to depress ATTD of DM in cellulose diets and increase AID of DM in pectin diets in this study, it is of paramount importance that if the intended value of the fibre source is to be achieved, one must decide when to take butter or fatty meat products v. vegetable oil-enriched foods. Among vegetable oils, palm oil could be an exception because of its high unsaturated FA content. More work is needed to investigate the interactions between DF solubility and lipid saturation using natural sources of fibre and at levels of inclusion for a practical dietary intervention that would be feasible for implementation in real-life human and swine feeding situations.
In conclusion, dietary inclusion of cellulose and pectin differently modulates the conditions in different segments of the GIT, thereby altering the apparent digestibility and flows of gastrointestinal FA. The interaction between DF and lipid types modulates digestibility of lipids and FA flows but differs for saturated and unsaturated FA and varies in different gastrointestinal segments. The digestibility of lipids is depressed and FA flows are increased in pectin-containing diets but more in diets supplemented with beef tallow than in all cellulose-containing diets. More work is needed to investigate how different fibre sources modulate conditions in different segments of the GIT and promote the endogenous losses of FA or microbial production or biotransformation of dietary and/or endogenous lipids.
Acknowledgements
The authors wish to thank DuPont Industrial Biosciences (Danisco UK Ltd) for financially supporting this research project. The authors are also grateful to the technical staff at the Department of Animal Biosciences at University of Guelph for postsurgical animal care (Douglas C. Wey) and in particular Wilfredo D. Mansilla and Cuilan L. Zhu for conducting surgeries.
The authors’ contributions are as follows: S. P. N., E. K., C. F. M. d. L. and C. M. N. designed the study. S. P. N. conducted the animal study, the statistical analysis and wrote the manuscript as part of his graduate studies. E. K., M. C. W., N. A., and C. M. N. critically reviewed the manuscript. C. M. N. was the principal investigator who supervised all aspects of the study. All the authors read, edited and approved the final manuscript.
The authors declared that there are no conflicts of interest.











