Cardiometabolic syndrome increases the risk of developing CVD and type 2 diabetes mellitus( Reference Alberti, Eckel and Grundy 1 ). Epidemiological and clinical studies have demonstrated the beneficial effects of tree nut and peanut consumption on the risk of CVD and its co-morbidities( Reference O'Neil, Keast and Nicklas 2 – Reference Jaceldo-Siegl, Haddad and Oda 4 ). Investigations of two independent cohorts of 76 464 women and 42 498 men have shown a 20 % reduction in total mortality in individuals who consumed nuts seven or more times a week v. those who did not consume nuts( Reference Bao, Han and Hu 5 ). Reductions in CVD mortality ( − 25 %) and death due to heart disease ( − 29 %) have also been reported for individuals who consumed nuts five times a week v. those who did not consume nuts( Reference Bao, Han and Hu 5 ). In addition, Jaceldo-Siegl et al. ( Reference Jaceldo-Siegl, Haddad and Oda 4 ) demonstrated a significant inverse association (P< 0·01) of high tree nut consumption with the prevalence of obesity in a group of 803 adults; a 46 % reduction in the prevalence of obesity was observed in high-tree nut consumers v. low-tree nut consumers.
Pistachios are a source of monounsaturated and polyunsaturated fats, as well as bioactive compounds, including plant sterols( Reference Phillips, Ruggio and Ashraf-Khorassani 6 ). The report of the 2010 Dietary Guidelines Advisory Committee states that plant sterols may contribute to the cholesterol-lowering effect reported for plant-based diets; the sterols compete with dietary cholesterol for absorption within the intestinal lumen( 7 ). Subsequently, endogenous cholesterol synthesis is increased, which in turn up-regulates LDL receptor expression and LDL removal from the circulation, resulting in a decrease in LDL levels( Reference Gylling, Plat and Turley 8 ).
In contrast to low-fat diets, moderate-fat diets that include nuts and other plant-based foods reduce LDL levels and typically increase HDL levels( Reference Sabaté, Oda and Ros 9 , Reference Appel, Sacks and Carey 10 ); additive effects have been observed with the inclusion of legumes, seeds, grains and nuts( Reference Appel, Sacks and Carey 10 ). However, questions remain regarding the mechanistic effects of the bioactive components of tree nuts and peanuts on the emerging markers of CVD risk. Mechanisms by which bioactive components in pistachios affect these emerging markers are also not known.
In individuals with insulin resistance, there is increased synthesis of VLDL, which results in increased TAG levels( Reference Zavaroni, Bonora and Pagliara 11 ) and decreased HDL levels( Reference Karhapää, Malkki and Laakso 12 ). The TAG:HDL ratio, a surrogate marker of insulin resistance, has been shown to predict an increased risk of hypertension and type 2 diabetes, as well as all-cause mortality in women with myocardial ischaemia( Reference McLaughlin, Abbasi and Cheal 13 – Reference Onat, Can and Kaya 15 ). In addition, decreased insulin sensitivity adversely affects LDL and HDL particle size. In a study carried out by Garvey et al. ( Reference Garvey, Kwon and Zheng 16 ), insulin resistance was found to be significantly associated with a decrease in LDL and HDL particle size, as well as increases in the levels of small and dense LDL (sdLDL) and small HDL particles, and a reduction in the levels of large LDL particles. In a study carried out by Irving et al. ( Reference Irving, Nair and Srinivasan 17 ) in non-diabetic subjects, low insulin sensitivity, cardiorespiratory fitness and truncal fat mass were all found to be associated with increases in the number of sdLDL and small HDL particles, as well as decreases in average LDL and HDL particle size; TAG was also found to be a strong predictor of lipoprotein levels and size. The effects of diet on lipoprotein particle size are important as sdLDL and the HDL subclasses have been shown to predict CVD risk better than LDL and HDL levels alone( Reference Krauss 18 – Reference Sharrett, Ballantyne and Coady 21 ).
Elevated levels of sdLDL are associated with an increased risk of coronary artery disease( Reference Gardner, Fortmann and Krauss 22 ). sdLDL particles are highly atherogenic primarily due to their increased susceptibility to oxidation and their ease of entry into the arterial wall( Reference Galeano, Al-Haideri and Keyserman 23 ). HDL are also heterogeneous and their subclasses are differentially associated with CVD risk( Reference Asztalos, Collins and Cupples 24 – Reference Asztalos, de la Llera-Moya and Dallal 26 ). Results obtained in the Framingham Offspring Study have demonstrated that low α-1 and α-2 HDL levels are stronger predictors of CHD risk than HDL levels alone( Reference Asztalos, Cupples and Demissie 25 ). Furthermore, the α-1 and α-2 subclasses of HDL are more functional in reverse cholesterol transport and promote atheroregression( Reference Fielding and Fielding 27 , Reference Rothblat and Phillips 28 ).
Recent advances in the development of ex vivo methodologies for assessing HDL functionality (serum cholesterol efflux capacity determination) provide an approach to evaluate the effects of pistachios on the lipid/lipoprotein profile and their association with reverse cholesterol transport. In the present study, we evaluated the effects of test diets that varied in the levels of total, monounsaturated and polyunsaturated fats provided by pistachios on the LDL and HDL subclasses, the TAG:HDL ratio, and serum cholesterol efflux capacity as a strategy for identifying mechanisms that explain how consumption of pistachios beneficially affects cardiometabolic status.
Experimental methods
Study design, participants and diet design
Plasma and serum samples obtained from the participants of our previous study( Reference Gebauer, West and Kay 29 ) were used for the analyses described herein. Briefly, otherwise healthy, non-smoking men (n 10) and women (n 18) with elevated fasting LDL levels ( ≥ 2·86 mmol/l, 110 mg/dl) completed a three-period, randomised, cross-over, controlled-feeding study( Reference Gebauer, West and Kay 29 ). Previous sample size calculation( Reference Gebauer, West and Kay 29 ) was based on effect sizes reported by Jenkins et al. ( Reference Jenkins, Kendall and Marchie 30 ) and Sabaté et al. ( Reference Sabaté, Haddad and Tanzman 31 ). Additional inclusion criteria were as follows: TAG levels < 3·94 mmol/l (349 mg/dl) and blood pressure < 160/90 mmHg; BMI between 21 and 35 kg/m2; fasting blood glucose levels ≤ 6·93 mmol/l (125 mg/dl). The baseline characteristics of the participants are given in Table 1. The participants were found to be not insulin resistant based on the homeostasis model of assessment-estimated insulin resistance (HOMA-IR) index ( < 2·5) and the TAG:HDL ratio ( < 1·53). Exclusion from the study was based on the following: an inability to adhere to the study protocol; using blood pressure-lowering or cholesterol/lipid-lowering medications or cholesterol/blood pressure-lowering products (psyllium, fish oil, soya lecithin and phyto-oestrogens); being pregnant or wishing to become pregnant 6 months before or during the study; lactating 6 weeks before or during the study; experiencing weight loss ≥ 10 % body weight 6 months before the study; following vegetarian or weight-loss diets; having any of the following conditions: stroke, diabetes, liver disease, kidney disease or autoimmune diseases.
Table 1 Baseline characteristics of the participants (Least-squares (LS) means with their standard errors, n 28)
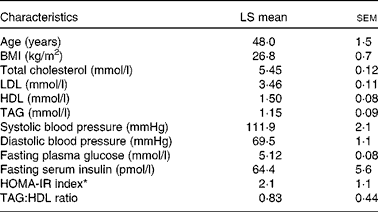
HOMA-IR, homeostasis model of assessment-estimated insulin resistance.
* HOMA-IR calculations were based on the method of Matthews et al. ( Reference Matthews, Hosker and Rudenski 35 ).
We used a controlled-feeding design and all meals were provided to the participants; energy levels were individualised for the maintenance of body weight. The macronutrient profile of the experimental diets is summarised in Table 2. After 2 weeks on a typical Western diet (35 % total fat (TF) and 11 % SFA), the participants were assigned to a balanced order sequence of three test diets. Each test diet was provided for 4 weeks followed by a 2-week compliance break. The following test diets were used: a lower-fat control diet (control; 25 % TF and 8 % SFA); a diet that provided 10 % of energy from pistachios (one serving of pistachios/d (1PD); 30 % TF and 8 % SFA); a diet that provided 20 % of energy from pistachios (two servings of pistachios/d (2PD); 34 % TF and 8 % SFA). Energy from carbohydrates was replaced with energy from pistachios. Salted, roasted pistachios (50 % of the daily dose) were consumed as snacks instead of baked potato chips and pretzels. Unsalted pistachios were incorporated into recipes.
Table 2 Macronutrient composition of the diets (n 28)
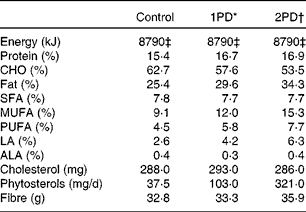
CHO, carbohydrate; LA, linoleic acid; ALA, α-linolenic acid.
* 1PD represents one serving (32–63 g or 1·5 oz) of pistachios per d (10 % energy from pistachios).
† 2PD represents two servings (63–126 g or 3·0 oz) of pistachios per d (20 % energy from pistachios).
‡ Equivalent to 8786 kJ (2100 kcal) of dietary energy.
Adherence to the study protocol was good, as demonstrated by daily compliance questionnaires. In addition, plasma β-sitosterol levels increased dose dependently with the inclusion of pistachios, consistent with dietary approximations, as β-sitosterol is the predominant sterol found in pistachios. The weight of the participants was recorded daily (Monday through Friday). Diets were isoenergetic, and there were no significant differences in pre-treatment and post-treatment means for either body weight (P>0·05) or BMI (P>0·05)( Reference Gebauer, West and Kay 29 ). No significant differences were also observed in body weight or BMI when comparing participants who consumed the control diet with those who consumed the pistachio diets (P>0·05)( Reference Gebauer, West and Kay 29 ).
The participants were instructed to maintain the intensity, frequency and duration of their habitual physical activity for the duration of the study. The participants were required to complete daily and weekly monitoring forms that were verified by the study coordinator. The daily monitoring form contained a ‘Comments’ section where the participants could record any physical activity outside of their usual routine. They were then specifically asked about their deviations in physical activity and were reminded to maintain their usual physical activity regimen.
The present study was approved by the Institutional Review Board of The Pennsylvania State University. All participants provided signed informed consent. Among the participants, one was unable to adhere to the protocol and subsequently withdrew from the study.
Analytical methods
Lipoprotein subclasses
Lipoprotein subclass analyses were conducted at the Boston Heart Diagnostics laboratory (Framingham, MA, USA). For sdLDL analysis, large, buoyant and other apoB-containing lipoproteins were first removed from the plasma by filtration after the formation of aggregates with a polyanion and divalent cation-based reagent, and sdLDL levels were then determined using a Cobas 6000 analyser (Roche), with reagents obtained from Denka-Seiken Company Limited, as described previously( Reference Ai, Otokozawa and Asztalos 32 ). The levels of HDL subclasses were determined by immunoblotting with prior separation using two-dimensional, non-denaturing PAGE as described previously( Reference Asztalos, Cupples and Demissie 25 ). Lipoprotein(a) levels were determined using ELISA as described elsewhere( Reference Lamon-Fava, Marcovina and Albers 33 ).
Measures of insulin sensitivity and inflammation
Methods used for determining fasting plasma glucose levels and fasting serum insulin levels have been described previously( Reference Gebauer, West and Kay 29 ). Briefly, fasting glucose levels were measured using an immobilised biosensor within the YSI 2300 STAT Plus Glucose & Lactate Analyzer (Yellow Springs Instruments Inc.). Insulin levels were measured using RIA with 125I-labelled human insulin and a human insulin antiserum (Linco Research, Inc.). Serum C-reactive protein (CRP) levels were measured using latex-enhanced immunonephelometry (Quest Diagnostics; assay CV < 8 %), and clinically significant limits were based on the 2003 AHA/CDC Scientific Statement on Markers of Inflammation and Cardiovascular Disease( Reference Pearson, Mensah and Alexander 34 ). Fasting plasma glucose levels and fasting serum insulin levels were determined at the Penn State Hershey Medical Center (Hershey, PA, USA). HOMA-IR index calculations were based on the formula proposed by Matthews et al. ( Reference Matthews, Hosker and Rudenski 35 ) using conventional units.
Serum cholesterol efflux capacity
Cholesterol efflux capacity was determined at Vascular Strategies LLC. Serum HDL samples (apoB-depleted serum) were prepared from individual serum samples by precipitation of apoB-containing lipoproteins using polyethylene glycol. Briefly, for each serum sample, 100 parts serum were mixed with forty parts polyethylene glycol (20 %, v/v, in glycine buffer, pH 7·4). The samples were then incubated at room temperature for 20 min and centrifuged at 10 000 rpm for 30 min at 4 °C. The supernatant containing serum HDL was collected and used for the determination of cholesterol efflux capacity.
Cholesterol efflux capacities of serum HDL samples were determined as described in detail elsewhere( Reference Mweva, Paul and Cambillau 36 , Reference de la Llera Moya, Drazul-Schrader and Asztalos 37 ). In brief, global and ATP-binding cassette transporter A1 (ABCA1)-mediated cholesterol effluxes were measured using J774 mouse macrophage cells in the presence and/or absence of cyclic AMP. For all assays, the cells were pre-incubated with [3H]cholesterol and acyl-CoA:cholesterol acyltransferase inhibitor Sandoz 58-035 (Sigma-Aldrich) overnight; cells were not preloaded with mass cholesterol. The cells were then incubated overnight in 0·2 % bovine serum albumin with or without 8-(p-chlorophenylthio)-cyclic AMP. After washing, the cells were incubated for 4 h with the serum HDL samples (apoB-depleted serum) added at 2·8 % (v/v). [3H]Cholesterol released into serum after incubation with the cells for 4 h was measured by liquid scintillation counting. Cholesterol efflux is expressed as the radiolabel released as a percentage of [3H]cholesterol within the cells before the addition of serum. All efflux values were corrected by subtracting the small amount of radioactive cholesterol released from the cells incubated with serum-free media. ABCA1-dependent efflux from J774 cells was determined as the difference in efflux from cyclic AMP-treated cells and that from untreated cells.
Circulating sterols
The levels of β-sitosterol, campesterol, desmosterol and lathosterol were measured using GC as described previously( Reference Matthan, Giovanni and Schaefer 38 ); analyses were carried out at the Boston Heart Diagnostics laboratory (Framingham, MA, USA). β-Sitosterol and campesterol were used as markers of dietary sterol absorption, while desmosterol and lathosterol were used as markers of endogenous cholesterol synthesis. As these sterols are primarily transported in the LDL fraction, their levels are expressed as a ratio to total cholesterol( Reference van Himbergen, Matthan and Resteghini 39 ).
Statistical analysis
Data were tested for normality and transformed where appropriate. All data, except those obtained for α-2, α-3 and α-4 HDL, fasting serum insulin and lipoprotein(a), were log-transformed; data obtained for lipoprotein(a) were transformed using the square root scale. The effects of diet were analysed by comparing the levels of each endpoint at the end of the control treatment with levels measured after the consumption of the two pistachio diets. Treatment differences were analysed using mixed models (SAS version 9.2; SAS Institute Inc.). Diet, period and diet × period interaction were entered as fixed effects; subject was a random effect. No significant diet × period interactions were observed for any variable, except for α-4 HDL; no significant effects of diet were observed on this variable. Changes from baseline were calculated for the HOMA-IR index. We examined whether change in LDL or sdLDL levels was predicted by change in serum β-sitosterol levels for the 2PD dietary treatment group; change scores were calculated for this group (values recorded at the end of the dietary treatment − those recorded after the control treatment). We also estimated whether change in sdLDL levels was related to change in TAG levels; change scores were calculated for the 2PD dietary treatment group (values recorded at the end of the dietary treatment − those recorded after the control treatment). As CRP is a strong predictor of CVD and the metabolic syndrome( Reference Hirschfield and Pepys 40 , Reference Rifai 41 ), we further determined whether there was an association between CRP status (high: ≥ 103μg/l v. low: < 103μg/l) at baseline and the effects of diets on cholesterol efflux capacity. Significant effects (P≤ 0·05) were evaluated using Tukey's tests. Data are reported as least-squares means with their standard errors.
Results
Lipoprotein subclasses
A significant effect of diet was observed on sdLDL levels (P= 0·001); compared with the control and 1PD diets, the 2PD diet significantly reduced sdLDL levels (P= 0·001 and P= 0·03, respectively; Table 3). Furthermore, reductions in sdLDL levels were positively associated with reductions in TAG levels following the 2PD dietary treatment v. the control treatment (r 0·42, P= 0·03). An increase in the levels of α-1 and α-2 HDL was observed with the inclusion of pistachios (P= 0·073 and P= 0·056, respectively; Table 3). No significant effects of diet were observed on preβ-1 HDL, α-3 HDL, α-4 HDL or lipoprotein(a) levels (Table 3); a significant diet × period interaction (P= 0·007) was observed for α-4 HDL levels.
Table 3 Effects of pistachio inclusion on lipoprotein subclasses (Least-squares (LS) means with their standard errors, n 28)

sdLDL, small and dense LDL.
* 1PD represents one serving (32–63 g or 1·5 oz) of pistachios per d (10 % energy from pistachios).
† 2PD represents two servings (63–126 g or 3·0 oz) of pistachios per d (20 % energy from pistachios).
‡ Tukey's adjustments were used for comparisons among the dietary treatment groups.
Cardiometabolic measures
The assessment of the TAG:HDL ratio, a marker of insulin resistance (Table 4), revealed a significant effect of diet (P= 0·041), and a significant reduction was observed in this ratio following the 2PD dietary treatment v. the control treatment (P= 0·036); the pistachio diets minimised the increase in the TAG:HDL ratio from baseline compared with the lower-fat control diet( Reference Gebauer, West and Kay 29 ). However, no significant effect of diet was observed on the HOMA-IR index (P= 0·71; Table 4). Change in the HOMA-IR index from baseline also did not differ among the dietary treatment groups (P= 0·994). No significant differences were observed in either fasting plasma glucose levels or fasting serum insulin levels among the dietary treatment groups.
Table 4 Effects of pistachio inclusion on the TAG:HDL ratio, fasting plasma glucose levels, fasting serum insulin levels, and the homeostasis model assessment-estimated insulin resistance index (HOMA-IR) (Least-squares (LS) means with their standard errors, n 28)
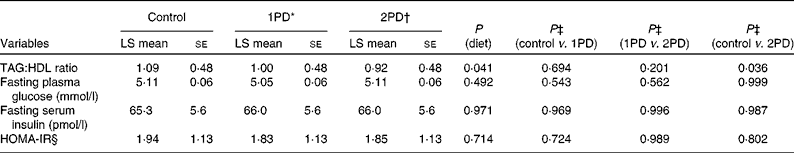
* 1PD represents one serving (32–63 g or 1·5 oz) of pistachios per d (10 % energy from pistachios).
† 2PD represents two servings (63–126 g or 3·0 oz) of pistachios per d (20 % energy from pistachios).
‡ Tukey's adjustments were used for comparisons among the dietary treatment groups.
§ HOMA-IR calculations were based on the method of Matthews et al. ( Reference Matthews, Hosker and Rudenski 35 ).
Serum cholesterol efflux capacity
No significant effects of diet were observed on ABCA1-mediated serum cholesterol efflux capacity (P= 0·213) or global serum cholesterol efflux capacity (P= 0·553; Table 5). After adjusting for baseline CRP status, however, significant diet × CRP status interactions were observed for both ABCA1-mediated serum cholesterol efflux capacity (P= 0·016) and global serum cholesterol efflux capacity (P= 0·029) (Table 6). A significant difference was observed in ABCA1-mediated serum cholesterol efflux capacity between participants consuming the 2PD diet (9·89 (se 0·75) %) and those consuming the 1PD diet (7·35 (se 0·74) %) within the low-CRP status group (P= 0·0158). A difference was also observed in global cholesterol efflux capacity between participants consuming the 2PD diet (17·55 (se 1·06) %) and those consuming the 1PD diet (14·73 (se 1·06) %) within the low-CRP status group (P= 0·076).
Table 5 Effects of pistachio inclusion on ATP-binding cassette transporter A1 (ABCA1)-mediated serum cholesterol efflux capacity (ABCA1 efflux) and global serum cholesterol efflux capacity (global efflux) (Least-squares (LS) means with their standard errors, n 28)
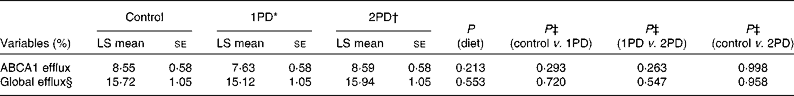
* 1PD represents one serving (32–63 g or 1·5 oz) of pistachios per d (10 % energy from pistachios).
† 2PD represents two servings (63–126 g or 3·0 oz) of pistachios per d (20 % energy from pistachios).
‡ Tukey's adjustments were used for comparisons among the dietary treatment groups.
§ Diet × period interaction (P= 0·023).
Table 6 Moderation of the effects of pistachio inclusion on ATP-binding cassette transporter A1 (ABCA1)-mediated serum cholesterol efflux capacity (ABCA1 efflux) and global serum cholesterol efflux capacity (global efflux) by C-reactive protein (CRP) status (n 28)
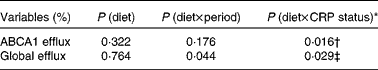
* Test for an interaction between pistachio inclusion and baseline CRP status (high: ≥ 103μg/l or low: < 103μg/l) for ABCA1-mediated and global serum cholesterol efflux capacities.
† Significant difference between those consuming two servings/d (9·89 (se 0·75) %) and those consuming one serving/d (7·35 (se 0·74) %) within the low-CRP status group (P= 0·016).
‡ Trend for a difference between those consuming two servings/d (17·55 (se 1·06) %) and those consuming one serving/d (14·73 (se 1·06) %) within the low-CRP status group (P= 0·076).
Circulating sterols
Significant effects of diet were observed on β-sitosterol (P< 0·0001) and campesterol (P= 0·009) levels. A significant increase in β-sitosterol levels was observed following the 1PD and 2PD dietary treatments v. the control treatment (P< 0·0001 for both; Table 7). A significant increase in β-sitosterol levels was also observed following the 2PD dietary treatment v. the 1PD dietary treatment (P= 0·0002). A significant decrease in campesterol levels was observed following the 2PD dietary treatment v. the control treatment (P= 0·009) and following the 1PD dietary treatment v. the control treatment (P= 0·051) (Table 7). Given the significant reductions in sdLDL levels observed in the present study and reductions in LDL levels reported previously (P< 0·001)( Reference Gebauer, West and Kay 29 ), we also evaluated whether increasing levels of β-sitosterol played a role in these reductions. Pearson's correlation analyses revealed no significant associations between reductions in sdLDL (r 0·26, P= 0·20) and LDL (r 0·30, P= 0·13) levels and increases in β-sitosterol levels on comparing the control and 2PD dietary treatment groups. No significant differences were observed in desmosterol (P= 0·14) or lathosterol (P= 0·15) levels among the dietary treatment groups (Table 7).
Table 7 Effects of pistachio inclusion on the levels of β-sitosterol and campesterol (markers of sterol absorption) and desmosterol and lathosterol (markers of endogenous cholesterol synthesis) (Least-squares (LS) means with their standard errors, n 28)
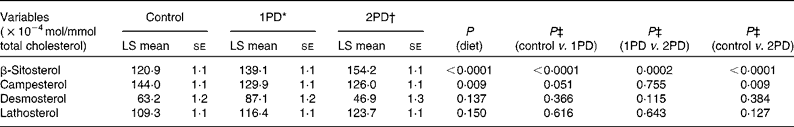
* 1PD represents one serving (32–63 g or 1·5 oz) of pistachios per d (10 % energy from pistachios).
† 2PD represents two servings (63–126 g or 3·0 oz) of pistachios per d (20 % energy from pistachios).
‡ Tukey's adjustments were used for comparisons among the dietary treatment groups.
Discussion
In the present study, the inclusion of pistachios in a heart-healthy diet as a strategy for increasing TF levels was found to favourably reduce sdLDL levels. The reduction in sdLDL levels would be expected to confer an additional benefit with regard to CVD risk beyond the reduction in LDL levels. Our previous findings showed the beneficial effects of pistachio consumption on lipid and lipoprotein profiles (Supplementary Table 1); specifically the 1PD and 2PD diets decreased LDL levels by − 9 and − 12 %, respectively( Reference Gebauer, West and Kay 29 ). sdLDL particles are associated with elevated TAG levels and lower HDL levels( Reference Reaven, Chen and Jeppesen 19 , Reference Austin, King and Vranizan 42 ), which are typically observed in individuals with dyslipidaemia and insulin resistance. We had previously reported a significant reduction in TAG levels with the inclusion of pistachios (1·40 (se 0·09) mmol/l for the lower-fat control diet, 1·28 (se 0·09) mmol/l for the 1PD diet, and 1·20 (se 0·09) mmol/l for the 2PD diet)( Reference Gebauer, West and Kay 29 ); the pistachio diets minimised the increase in TAG levels from baseline (1·15 (se 0·09) mmol/l) compared with the lower-fat control diet. In the present study, we found a significant correlation between reductions in both sdLDL and TAG levels, indicating a beneficial shift in the metabolic profile.
With a decrease in insulin sensitivity, a predominance of sdLDL particles is typically associated with a high TAG:HDL ratio( Reference Salazar, Carbajal and Espeche 43 ). In comparison with the lower-fat control diet, the pistachio diets resulted in decreases in both sdLDL levels and the TAG:HDL ratio; they also reduced increases in the TAG:HDL ratio from baseline when compared with the lower-fat control diet. Although the TAG:HDL ratio was still in the normal range in all participants ( < 1·53), further reductions may confer additional cardioprotective benefits. Our findings are important because we studied generally healthy individuals with elevated LDL levels who did not present with insulin resistance (as assessed by HOMA-IR scores) and yet observed beneficial effects on sdLDL levels and the TAG:HDL ratio in response to a moderate-fat diet containing pistachios. The TAG:HDL ratio is important because it incorporates two of the criteria for the metabolic syndrome and has been shown to predict insulin resistance, incident hypertension and diabetes( Reference McLaughlin, Abbasi and Cheal 13 , Reference Onat, Can and Kaya 15 , Reference Taskinen 44 ). The results of the present study demonstrate improvements in the TAG:HDL ratio, indicating that progression to insulin resistance can be slowed by consumption of a moderate-fat, heart-healthy diet containing pistachios.
Studies have shown that moderate-fat diets that contain tree nuts or peanuts increase HDL levels when compared with lower-fat diets( Reference Sabaté, Oda and Ros 9 ). In our previous study, we had also found beneficial effects on HDL levels with the inclusion of pistachios in the diet; there was a significant difference in HDL levels between those who consumed the 1PD diet and those who consumed the 2PD diet; there were no differences between those who consumed either the 1PD or the 2PD diet and those who consumed the lower-fat control diet. However, it must be noted that the mean HDL level at baseline in all participants was approximately 1·50 mmol/l, which is moderately high. The inclusion of pistachios (2PD) in the diet further attenuated decreases in HDL levels from baseline when compared with the lower-fat control diet( Reference Gebauer, West and Kay 29 ). Interestingly, we observed trends in the effects of diet on α-1 and α-2 HDL levels. These larger, cholesterol-rich HDL particles have been shown to enhance reverse cholesterol transport to promote atheroregression v. the relatively small and immature subclasses, which do not contribute to the regression of an atherosclerotic plaque( Reference Rothblat and Phillips 28 ). An ex vivo assay of serum cholesterol efflux capacity did not reveal differences among the dietary treatment groups. However, previous studies have indicated that circulating levels of CRP can predict the risk of atherosclerotic plaque formation and thrombotic events( Reference Hirschfield and Pepys 40 , Reference Rifai 41 ). When the effect of baseline CRP status was taken into account, we found significant increases in both ABCA1-mediated and global serum cholesterol efflux capacities following the 2PD dietary treatment v. the 1PD dietary treatment when baseline CRP values were < 103μg/l. These results indicate that HDL functionality may be increased with the inclusion of pistachios in the absence of low-grade inflammation. As such, effects of diet on cholesterol efflux capacity may be blunted in individuals with chronic systemic inflammation. Future clinical studies should consider inflammatory status for a more accurate description of the cardioprotective effects conferred by nut consumption.
There were no significant effects of diet on lipoprotein(a), indicating that dietary fat from pistachios may not influence this cardiometabolic marker. Although elevated lipoprotein(a) levels ( ≥ 1·07 μmol/l) are associated with an increased risk of CVD, and modestly associated with insulin resistance( Reference Luc, Bard and Arveiler 45 ), levels observed in the present study (lower-fat control, 0·89 (se 0·01) μmol/l; 1PD, 0·90 (se 0·01) μmol/l; 2PD, 0·89 (se 0·01) μmol/l) were below the cut-off associated with an increased risk.
As has been discussed, the 1PD and 2PD diets significantly decreased LDL levels ( − 9 and − 12 %, respectively) compared with the lower-fat control diet( Reference Gebauer, West and Kay 29 ). In the present study, β-sitosterol levels were elevated in the 1PD and 2PD dietary treatment groups v. the control group. In addition, β-sitosterol levels were inversely associated with campesterol levels in participants consuming the pistachio diets. Pistachios provide 200 mg of β-sitosterol and 10 mg of campesterol per 100 g( 7 ), which account for the shift in the plasma sterol profile. Thus, increases in β-sitosterol levels with the inclusion of pistachios confirm participants' adherence to the study protocol. Despite elevated β-sitosterol levels, decreased cholesterol absorption( Reference Normen, Dutta and Lia 46 , Reference Escurriol, Cofan and Serra 47 ) probably was not the primary factor contributing to the LDL-lowering effect observed following the 1PD and 2PD dietary treatments, as there were no significant associations between reductions in LDL and sdLDL levels and increases in β-sitosterol levels. This is not unexpected as the 1PD and 2PD diets provided only 103 and 321 mg phytosterols per d, which are appreciably less than the recommended intake of 2–3 g sterols/stanols per d for cholesterol-lowering effects to be observed( Reference Nguyen 48 ). Nonetheless, it is the combination of β-sitosterol levels and the fatty acid profile of the pistachio diets that most probably explains the cholesterol-lowering effects observed in the present study. Furthermore, the inclusion of pistachios may aid in the maintenance of whole-body cholesterol homeostasis( Reference Taverne, Richard and Couture 49 ), as evidenced by the absence of a change in desmosterol and lathosterol levels.
The present study has a few limitations. The duration of the study may have been too short to detect shifts in the lipoprotein subclass profile, particularly in the HDL subclasses. Other limitations include the lack of plasma samples for baseline lipoprotein subclass determinations, as well as waist circumference measurements for conclusive assessment of cardiometabolic status. Had we access to these data, we could have investigated the changes in subclass levels from baseline following the dietary treatments and evaluated how reductions in sdLDL levels and the TAG:HDL ratio are related to any change in waist circumference in participants over the course of the study. In addition, we observed effects of diet on cholesterol efflux capacity only in participants with low CRP status. Although this was not accounted for a priori, these results indicate that the traditional model for dietary treatment effects may not be as successful as in individuals with chronic systemic inflammation. Future studies should monitor the inflammatory status of participants at baseline and over the course of the study.
The present study provides new insights into the role of pistachios and their bioactive components that affect the markers of cardiometabolic syndrome. Collectively, we showed that inclusion of pistachios at 10–20 % energy per d as part of a heart-healthy, cholesterol-lowering diet decreases sdLDL levels, reduces the TAG:HDL ratio, modestly increases the levels of the functional HDL subclasses, and has beneficial effects on cholesterol efflux capacity (only in the lower-CRP status group), which in aggregate would be expected to decrease the risk of cardiometabolic syndrome. Based on our findings, we conclude that the inclusion of pistachios in a moderate-fat diet may prevent and slow the transition to CVD and diabetes. Further studies are required to directly evaluate whether the consumption of a heart-healthy, moderate-fat diet (that contains pistachios or other healthy fats) can prevent the onset of insulin resistance in individuals at an increased risk of cardiometabolic syndrome.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514001561
Acknowledgements
Clinical services were provided by the General Clinical Research Center of The Pennsylvania State University (University Park, PA, USA). Biochemical analyses were conducted at the Boston Heart Diagnostics (formerly Boston Heart Laboratory), Framingham, MA, USA, and Vascular Strategies LLC, Plymouth Meeting, PA, USA.
The authors thank Dr Heidi Collins and Dr Steven Adelman of Vascular Strategies LLC; Dr Ernest Schaefer of Boston Heart Diagnostics; and Katherine A. Sauder of The Pennsylvania State University for providing technical assistance.
The California Pistachio Commission of Fresno California and the Western Pistachio Association (now the American Pistachio Growers) provided primary funding for the study. The study was partially supported by NIH grant M01 RR 10732. C. D. K. was supported by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada.
The authors' contributions are as follows: S. D. H., S. G. W. and P. M. K.-E. formulated the research questions; S. K. G., C. D. K., S. G. W. and P. M. K.-E. designed the original clinical study; S. K. G., C. D. K., S. G. W. and P. M. K.-E. conducted the original clinical study; S. D. H., S. G. W. and P. M. K.-E. analysed the data. All authors contributed to the writing of the manuscript.
P. M. K.-E. and S. G. W. received research grants and travel support from the Western Pistachio Association (now the American Pistachio Growers). Other authors have no conflicts of interest to disclose.











