Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are characterised by chronic inflammation of the gastrointestinal tract. The incidence of IBD is greater in developed countries, such as Western countries; however, its incidence in East Asian countries such as Japan, Korea and China is also increasing( Reference Asakura, Nishiwaki and Inoue 1 , Reference Molodecky, Soon and Rabi 2 ). The aetiology of IBD most likely involves a complex interaction of genetic, environmental and immunoregulatory factors, but its precise pathogenesis remains unclear( Reference Xavier and Podolsky 3 ). Dietary factors, such as an increased prevalence of the ‘Western’ diet, which is high in fat and protein but low in fruits and vegetables, may be associated with the increase in the number of patients with IBD. Hou et al. ( Reference Hou, Abraham and El-Serag 4 ) have reported that high dietary intake of fat and meat was associated with an increased risk for IBD, and high fibre, fruit and vegetable intake was associated with a decreased risk for IBD. Thus, dietary fibre might be involved in the pathogenesis of IBD and might be an important constituent of a therapeutic strategy for IBD, although low-fibre diets are frequently recommended for IBD patients with strictures( Reference Lewis and Fisher 5 ).
Partially hydrolysed guar gum (PHGG) is a water-soluble dietary fibre prepared from guar gum and used as a food stabiliser and thickener( Reference Yoon, Chu and Raj Juneja 6 ). PHGG has been reported to improve symptoms associated with both constipation- and diarrhoea- predominant forms of irritable bowel syndrome( Reference Giannini, Mansi and Dulbecco 7 ). In addition, dietary PHGG has been demonstrated to be beneficial in the treatment of cholera( Reference Alam, Ashraf and Sarker 8 ), small intestinal bacterial overgrowth( Reference Furnari, Parodi and Gemignani 9 ), paediatric functional gastrointestinal disorders( Reference Romano, Comito and Famiani 10 ) and the metabolic syndrome-related functions such as aberrant lipid and glucose metabolism( Reference Yasukawa, Naito and Takagi 11 , Reference Dall’Alba, Silva and Antonio 12 ). With regard to intestinal inflammation, PHGG treatment has been shown to significantly reduce colonic mucosal damage in a dextran sulphate sodium-induced colitis model, a widely accepted murine model for UC( Reference Naito, Takagi and Katada 13 ).
PHGG, similar to other dietary fibres, is not digested in the upper gastrointestinal tract and is fermented by colonic bacteria, resulting in the formation of SCFA, particularly butyrate. Ohashi et al. ( Reference Ohashi, Sumitani and Tokunaga 14 ) have demonstrated that PHGG consumption stimulated the growth of butyrate-producing bacteria and, interestingly, Bifidobacterium in the intestine. Therefore, PHGG should have beneficial effects on host health through the alteration of colonic microbiota and production of SCFA. However, the mechanisms underlying the alteration of colonic microbiota and production of colonic SCFA after PHGG treatment have not been fully investigated.
In this study, we quantified selected bacterial taxa and SCFA composition in murine caecal faeces after PHGG administration to study the effects of PHGG on colonic mucosal inflammation in a murine 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model, which shares immunological and pathological features with human CD.
Methods
Animals
Male C57BL/6 mice (n 48) aged 7 weeks were obtained from SHIMIZU Laboratory Supplies Co. Ltd. The mice were caged individually in a room kept at 18–24°C and 40–70 % relative humidity, with a 12 h light–12 h dark cycle and ad libitum access to food and drinking water. All animals were randomised into groups receiving either a PHGG or an American Institute of Nutrition (AIN)-93G diet (Nihon Clea). The animals receiving a PHGG diet were exposed to 5 % PHGG for 2 weeks before the induction of colitis with TNBS. All animals were maintained and experimental procedures carried out in accordance with National Institutes of Health (NIH) guidelines for the use of experimental animals. All experimental protocols were approved by the Animal Care Committee of Kyoto Prefectural University of Medicine.
Induction of colitis
To induce colitis, 100 mg/kg TNBS (Sigma-Aldrich Japan) dissolved in 30 % ethanol was intrarectally administered to lightly anaesthetised (ketamine/xylazine) mice via a catheter. The control mice were intrarectally administered with 30 % ethanol. A duration of 3 d after TNBS administration, the animals were killed, and the colon was removed and examined. The severity of colonic damage was graded according to previously established criteria( Reference Harusato, Naito and Takagi 15 , Reference Takagi, Naito and Mizushima 16 ). The presence of visible damage, serosal adhesions, diarrhoea, strictures and bowel wall thickening was included in the macroscopic grading. All scoring was performed by the same individual under blinded conditions to prevent observer bias.
Histologic evaluation
Specimens of the distal colon were fixed in 10 % neutral buffered formalin. The severity of histological damage was graded according to previously established criteria( Reference Elson, Beagley and Sharmanov 17 ). In brief, the histological scores represented the presence and extent of inflammation, damage, and regeneration in colitis. After fixation, the specimens were embedded in paraffin, divided into 4-μm sections, and stained with haematoxylin–eosin.
Measurement of myeloperoxidase activity
Tissue-associated myeloperoxidase (MPO) activity was measured as an index of neutrophil accumulation in the colonic mucosa by a modification of the method of Grisham et al. ( Reference Grisham, Hernandez and Granger 18 ). The colonic mucosa (4-cm length of distal colon) was scraped off using two glass slides and was homogenised with 1·0 ml of PBS in a Teflon Potter-Elvehjem homogenizer (As One Corp.). The resultant mucosal homogenates were centrifuged at 20 000 g for 15 min at 4°C to separate out the insoluble cellular debris. The pellet was re-homogenised in 0·3 ml of 0·05 mol potassium phosphate buffer (pH 5·4) containing 0·5 % hexadecyltrimethylammonium bromide. The samples were centrifuged at 20 000 g for 15 min at 4°C, and the supernatants were saved. MPO activity was assessed by measuring the H2O2-dependent oxidation of 3,3',5,5'-tetramethylbenzidine. One unit of enzyme activity was defined as the amount of MPO that caused a change in the 1·0/min absorbance at 460 nm and 37°C. The total protein in the tissue homogenates was measured with a Bio-Rad Protein Assay kit (Bio-Rad Laboratories) according to the manufacturer’s protocol.
Measurement of the intestinal TNF-α mRNA expression
The colonic mRNA expression of TNF-α and β-actin (internal control) was determined by real-time PCR according to a previously used protocol( Reference Higashimura, Naito and Takagi 19 ). Total RNA was isolated using the acid guanidinium–phenol–chloroform method with an Isogen kit (Nippon Gene Co. Ltd). The isolated RNA was stored at −80°C until use in real-time PCR. Later, 1 μg of extracted RNA was reverse-transcribed into first-strand complementary DNA (cDNA) using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR for TNF-α and β-actin was conducted with a 7300 Real-Time PCR system (Applied Biosystems) using the DNA-binding dye SYBR Green to detect the PCR products, using the following primers: for TNF-α, sense 5'-ATCCGCGACGTGGAACTG-3' and antisense 5'-ACCGCCTGGAGTTCTGGAA-3'; and for β-actin, sense 5'-TATCCACCTTCCAGCAGATGT-3' and antisense 5'-AGCTCAGTAACAGTCCGCCTA-3'. mRNA expression was quantified relative to β-actin expression.
Determination of the intestinal TNF-α content
We determined the concentration of TNF-α in the supernatant of mucosal homogenates using an ELISA kit (eBioscience Inc.), specific for mouse TNF-α, following a sandwich method. The assay was performed according to the manufacturer’s instructions. After colour development, optimal densities were measured at 450 nm with a microplate reader (Spectramax M2; Molecular Devices Corp.).
Analysis of caecal bacteria
Bacterial genomic DNA was extracted from caecal contents (approximately 50 mg), according to the method described by Godon et al. ( Reference Godon, Zumstein and Dabert 20 ). The 16S rRNA gene of the Clostridium coccoides group (Clostridium cluster XIVa), Clostridium leptum subgroup (Clostridium cluster IV), Clostridium cluster I, Clostridium cluster XI, Bacteroides fragilis group, Enterococcus, Lactobacillus and Enterobacteriaceae was quantified by real-time PCR performed with an MyiQ real-time PCR system (Bio-Rad). The reaction mixture (20 µl) contained 10 µl of the iQ SYBER Green Supermix (Bio-Rad), 0·5 µl of caecal DNA and 400 µmol/l of each primer. Previously described primers were used in this study( Reference Song, Liu and Finegold 21 – Reference Matsuki, Watanabe and Fujimoto 23 ). The thermal programme consisted of initial denaturation at 95°C for 3 min, followed by forty cycles of denaturation at 94°C for 10 s, annealing at optimum temperature for 30 s, elongation at 72°C for 40 s, and final elongation at 72°C for 5 min. Fluorescent products were detected at the last step of each cycle. Melting curve analysis of the product was performed after amplification to determine the specificity of the PCR. A plasmid containing a partial sequence of the 16 S rRNA gene identical to that of the targeted bacteria was constructed in our laboratory and used as standard DNA.
Analysis of faecal organic acids
A portion of the caecal contents (0·5 g) was suspended with 0·5 ml of 14 % perchloric acid to eliminate proteins. After centrifugation at 10 000 g at 4°C for 5 min, the supernatant was filtered through a cellulose–acetate membrane filter with 0·45-µm pores, and its organic acid content was analysed by ion-exclusion HPLC as described by Ushida & Sakata( Reference Ushida and Sakata 24 ).
Statistical analysis
Results are presented as means with their standard errors. Overall differences between groups were determined by one-way ANOVA. If the results of the one-way ANOVA were significant, differences between individual groups were analysed by the Bonferroni multiple comparison test. Histological scores were statistically analysed using the Mann–Whitney U test. For analysis of caecal bacteria, the copy number of targeted genes and concentration of organic acids were analysed using the Friedman test, followed by the Steel–Dwass method. Values of P<0·05 were considered significant. All analyses were performed using the GraphPad Prism 6 program (GraphPad Software Inc.) for Macintosh.
Results
Effect of partially hydrolysed guar gum on trinitrobenzene sulfonic acid-induced colonic damage
Macroscopic findings in the colon of mice exposed to TNBS indicated severe colitis with hyperaemia, oedema, thickening and ulceration. Pre-feeding treatment with 5 % PHGG reduced the size of the macroscopic lesion (Fig. 1(a)). The colonic damage score had increased significantly after TNBS administration, but this increase was significantly dampened by 5 % PHGG treatment (Fig. 1(b)). In addition, the degree of body weight loss after the induction of colitis with TNBS was significantly inhibited in 5 % PHGG-treated mice (Fig. 1(c)).
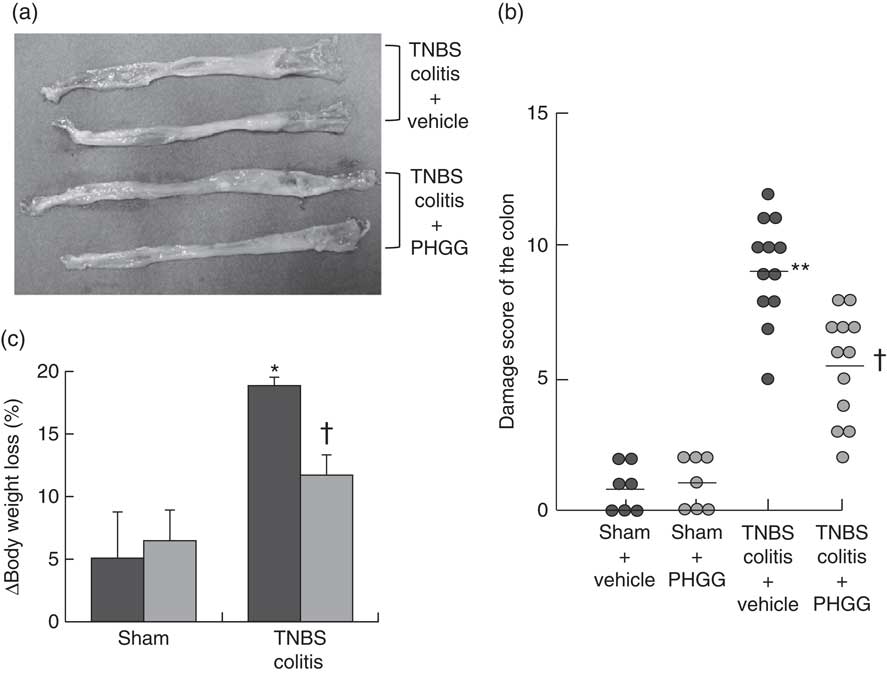
Fig. 1 Effects of partially hydrolysed guar gum (PHGG) on 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colonic damage. (a) Representative macroscopic findings in the colon on day 3 after the induction of injury by TNBS administration. TNBS induced severe colitis with hyperaemia, oedema, thickening, ulceration and necrosis, which were reduced in mice treated with PHGG. (b) Damage score of the colon of mice treated with PHGG until day 3 after the induction of colitis. Data are expressed as a scatter plot. ** P<0·01 compared with the sham group. † P<0·05 compared with the TNBS colitis group. (c) Decrease in body weight after the induction of TNBS colitis was significantly attenuated in mice treated with PHGG. * P<0·05 compared with the sham group. † P<0·05 compared with the TNBS-induced colitis group. ![]() , PHGG;
, PHGG; ![]() , TNBS.
, TNBS.
Fig. 2(a) shows typical histological features in the TNBS-only and 5 % PHGG-treated groups. Haematoxylin–eosin-stained colonic specimens of TNBS-only mice showed extensive ulcer formation with massive infiltration of inflammatory cells, which were predominantly neutrophils. In contrast, specimens of 5 % PHGG-treated mice showed lesser ulceration and infiltration of inflammatory cells. Further, the histological scores of 5 % PHGG-treated mice were significantly lower than those of TNBS-only mice (Fig. 2(b)).
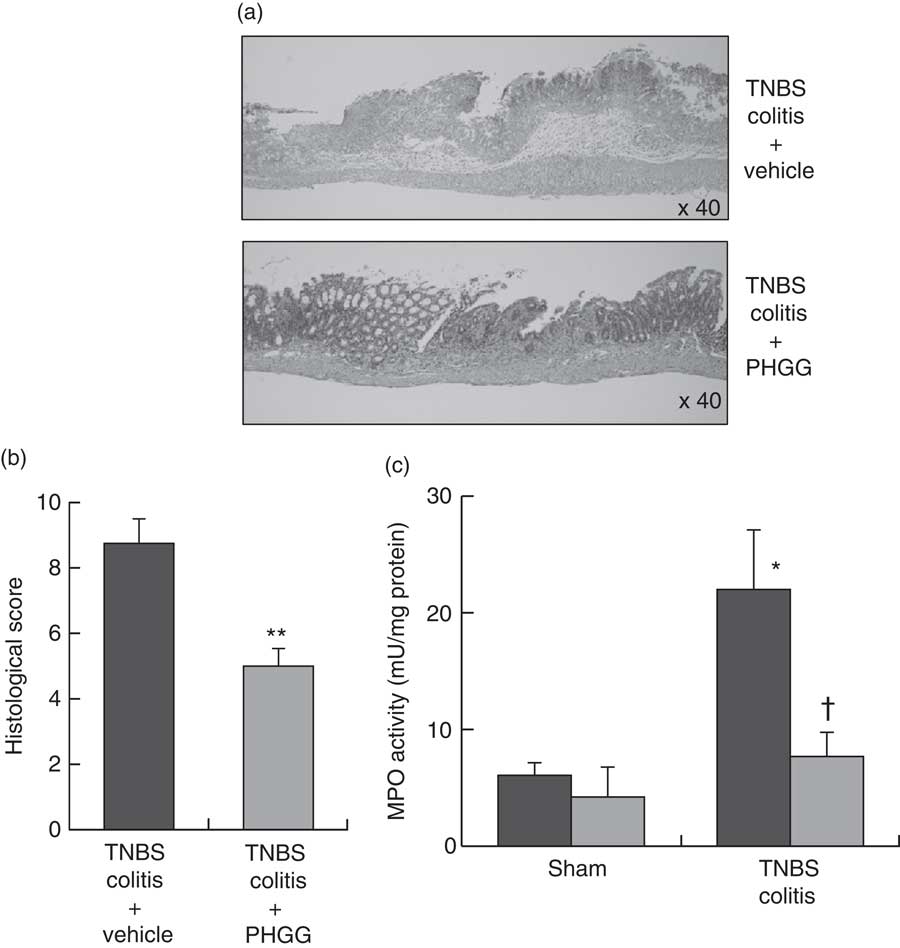
Fig. 2 Effects of partially hydrolysed guar gum (PHGG) on 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. (a) Histological appearance of the colon of mice administered only TNBS and of those treated with PHGG and administered TNBS. Magnification, 40×. Haematoxylin–eosin staining. (b) Histological score of the colon of mice administered only TNBS and of those treated with PHGG and administered TNBS. Values are means (n 5), with their standard errors. ** P<0·01 compared with vehicle-treated mice with TNBS-induced colitis. (c) Tissue-associated myeloperoxidase (MPO) activity as an index of neutrophil accumulation in the colonic mucosa. Values are means (n 7), with their standard errors. * P<0·05 compared with the sham group. † P<0·05 compared with the TNBS colitis group. ![]() , Vehicle;
, Vehicle; ![]() , PHGG.
, PHGG.
Effects of partially hydrolysed guar gum treatment on myeloperoxidase activity
Neutrophil accumulation was evaluated by measuring tissue-associated MPO activity in colonic mucosal homogenates. In the sham groups, there were no differences in the MPO activities of vehicle- and 5 % PHGG-treated mice. However, in the TNBS-treated groups, MPO activity in the colonic mucosa was markedly increased after TNBS administration compared with the sham groups. This increase after TNBS administration was significantly inhibited by treatment with 5 % PHGG (Fig. 2(c)).
Effect of partially hydrolysed guar gum treatment on TNF-α expression
To investigate the effect of PHGG treatment on TNF-α expression in the colonic mucosa, we analysed the colonic TNF-α mRNA expression and mucosal TNF-α protein content using ELISA. In the sham-operated groups, there were no significant differences between the vehicle- and 5 % PHGG-treated mice. However, the colonic expression of TNF-α mRNA was significantly increased in TNBS-treated mice, and this increase was significantly reduced by 5 % PHGG treatment (Fig. 3(a)). Similarly, the mucosal TNF-α protein content was significantly increased in TNBS-treated mice, and this increase was significantly attenuated by 5 % PHGG treatment (Fig. 3(b)).
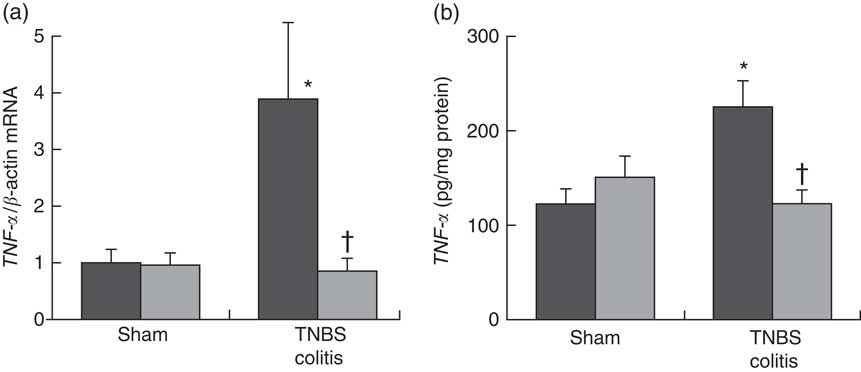
Fig. 3 Effect of partially hydrolysed guar gum (PHGG) on TNF-α expression in the colonic mucosa after TNBS-induced injury. (a) TNF-α mRNA expression in colonic mucosa. Values are means (n 7), with their standard errors. * P<0·05 compared with the sham group. † P<0·05 compared with the TNBS colitis group. (b) Concentration of TNF-α in the supernatant of mucosal homogenates. Values are means (n 7), with their standard errors. * P<0·05 compared with the sham group. † P<0·05 compared with the TNBS colitis group. ![]() , Vehicle;
, Vehicle; ![]() , PHGG.
, PHGG.
Effect of partially hydrolysed guar gum on the caecal bacterial profile
To determine whether PHGG treatment altered the caecal bacterial profile, we examined the variation in eight bacterial groups using real-time PCR. As shown in Table 1, compared with vehicle-treated mice, 5 % PHGG-treated mice showed significantly increased caecal proliferation of the C. coccoides group (Clostridium cluster XIVa), the C. leptum subgroup (Clostridium cluster IV) and the B. fragilis group. Conversely, 5 % PHGG treatment significantly reduced the proliferation of caecal Clostridium cluster XI compared with control diet.
Table 1 The alteration of colonic microbiota after treatment with 5 % partially hydrolysed guar gum (PHGG) (Mean values and standard deviations, seven mice per group)
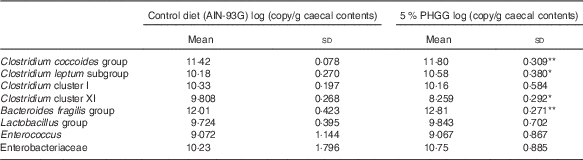
* P<0·05, ** P<0·01 v. control diet (AIN-93G).
Effect of partially hydrolysed guar gum on the caecal SCFA profile
To determine whether PHGG treatment altered the caecal SCFA profile, we determined the caecal SCFA content by using ion-exclusion HPLC (Table 2). PHGG treatment stimulated the production of acetic acid, propionic acid, butyric acid and succinic acid compared with the control diet. No differences were observed in the production of formic acid, lactic acid, isobutyric acid, isovaleric acid and valeric acid.
Table 2 The alteration of SCFA in the colonic lumen after treatment with 5 % partially hydrolysed guar gum (PHGG) (Mean values and standard deviations, seven mice per group)
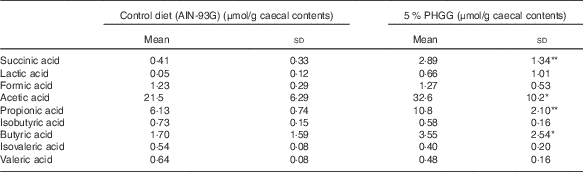
* P<0·05, ** P<0·01 v. control diet (AIN-93G).
Discussion
We demonstrated that treatment with PHGG ameliorated TNBS-induced colonic injury and inflammation in mice. In our study, colonic injury was assessed on the basis of the macroscopic damage and histological scores, and treatment with PHGG was found to significantly reduce colonic injury. In addition, we showed that the MPO activity and TNF-α mRNA and protein production in the intestinal mucosa were enhanced in TNBS-induced intestinal inflammation, and these increases were significantly reversed by PHGG treatment.
TNF-α has recently attracted attention for its key role in the pathogenesis of IBD, and TNF-α-blocking agents have been used as therapeutic agents for treating IBD worldwide( Reference Rutgeerts, Vermeire and Van Assche 25 ). Our results are consistent with those of a previous study that used a different murine colitis model to demonstrate that PHGG ameliorated murine colonic inflammation through inhibition of colonic mucosal TNF-α production( Reference Naito, Takagi and Katada 13 ). Although the detailed mechanism by which PHGG may inhibit TNF-α production remains to be elucidated, PHGG may have therapeutic potential for patients with IBD.
An important result of the present study is that the alerted profiles of caecal bacteria and SCFA can be identified after PHGG treatment. Recent studies have demonstrated that microbiota plays a role in the onset and progression of intestinal inflammation in IBD, and the therapeutic efficacy of antibiotics, probiotics and prebiotics in relation to their effects on microbiota has been noted in the treatment of IBD( Reference Sartor 26 ). With regard to the caecal bacterial profile, we classified the bacteria into the C. coccoides group (Clostridium cluster XIVa), the C. leptum subgroup (Clostridium cluster IV) and the B. fragilis group as SCFA-producing indigenous bacteria, Enterococcus as indigenous bacteria, Clostridium cluster I and XI, which contains well-known harmful bacteria including Clostridium difficile and Clostridium perfringens, the Lactobacillus group as lactate-producing bacterium, and Enterobacteriaceae as pathogenic bacteria, such as Escherichia coli. PHGG treatment significantly increased the concentration of the caecal C. coccoides group (Clostridium cluster XIVa), the C. leptum subgroup (Clostridium cluster IV), and the B. fragilis group and significantly decreased the concentration of caecal Clostridium cluster XI compared with the control diet.
Interestingly, Sokol et al.( Reference Sokol, Seksik and Furet 27 ) have shown that the C. leptum subgroup and C. coccoides group were less represented in patients with active IBD. Moreover, Ando et al.( Reference Andoh, Kuzuoka and Tsujikawa 28 ) have demonstrated that Clostridium cluster XIVa and IV were significantly decreased in patients with active-phase CD than in healthy individuals. Thus, a reduction in these bacteria may be associated with susceptibility to intestinal inflammation. Therefore, an increase in these bacteria by PHGG administration might lead to protective effects against intestinal inflammation. Koleva et al. ( Reference Koleva, Valcheva and Sun 29 ) have reported that chronic intestinal inflammation consistently correlated with the abundance of Clostridium cluster XI, which are well-known harmful bacteria, in HLA-B27 transgenic rats, a well-validated rodent model for IBD, and they focused on reducing Clostridium cluster XI to inhibit intestinal inflammation by treatment with inulin and fructo-oligosaccharides. In agreement with these data, in the present study, PHGG administration reduced Clostridium cluster XI, which correlated with a decrease in chronic intestinal inflammation.
Compositional alterations of the colonic microbiota may also change metabolic capacities of the gut bacteria( Reference De Preter, Machiels and Joossens 30 ). Colonic fermentation of carbohydrates results in the generation of SCFA, which are generally assumed to be beneficial for the host( Reference Macfarlane and Macfarlane 31 ). Reduced levels of faecal SCFA have previously been linked to a shift in the composition and metabolic activity of the colonic microbiota in IBD( Reference Sokol, Seksik and Furet 27 , Reference Andoh, Kuzuoka and Tsujikawa 28 ). Treatment with PHGG caused a significant increase in the caecal content of the SCFA-producing bacterial group, the C. coccoides group (Clostridium cluster XIV), the C. leptum group (Clostridium cluster IV) and the B. fragilis group. These results were consistent with those of previous experiments that indicated that PHGG stimulated butyrate-producing bacteria( Reference Ohashi, Sumitani and Tokunaga 14 ). Recent reports have demonstrated that Clostridium cluster XIV, Clostridium cluster IV, and the B. fragilis group promoted accumulation of CD4+ T regulatory (Treg) cells( Reference Atarashi, Tanoue and Shima 32 , Reference Round and Mazmanian 33 ). In addition, Smith et al.( Reference Smith, Howitt and Panikov 34 ) showed that three SCFA (propionate, acetate and butyrate), individually or in combination, derived from common bacterial metabolites increased the number of Treg cells in the large intestine. In our study, production of acetic acid, propionic acid and butyric acid was stimulated by PHGG treatment. The inhibition of TNBS-induced colitis by PHGG treatment might be due to the promotion of Treg cells, which are critical for limiting intestinal inflammation( Reference Atarashi, Umesaki and Honda 35 ); however, we could not fully investigate this issue in this study. Thus, dietary fibre stimulates the growth of intestinal bacteria such as Clostridium cluster XIV, Clostridium cluster IV, and the B. fragilis group and may enhance the microbial balance in the colon, because these bacteria produce SCFA and anti-microbial compounds that may protect against increased growth of potentially pathogenic bacteria such as C. perfringens and E. coli ( Reference Zhang, Li and Gan 36 ).
Our study includes potential limitations. Our investigation evaluated a limited list in intestinal microbiota and did not investigate several important bacteria including Bifidobacterium. In addition, we evaluated SCFA and microbiota after the development of TNBS-induced colitis. Therefore, further experiments would be required to elucidate these issues.
In summary, our results indicate that PHGG treatment inhibits intestinal inflammation in mice, which is associated with a significant decrease in TNF-α production and neutrophil infiltration in the intestinal mucosa. These effects may lead to changes in caecal bacterial and SCFA profiles. Although further studies are warranted to unravel the mechanisms underlying this phenomenon, the administration of PHGG has potential as a new therapeutic strategy for IBD.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) (C) to T. T. (no. 25460959) and Y. N. (no. 25460958) from the Japan Society for the Promotion of Science and by an Adaptable and Seamless Technology Transfer Program Through Target-Driven R&D (to Y. N.) grant from the Japan Agency for Medical Research and Development.
T. T. and Y. N. conceived this experiments; T. T., Y. H., C. U., K. M., K. Katada and K. Kamada performed the majority of in vivo experiments; K. U. and O. H. performed the majority of TNF expression experiments; Y. O. performed analysis of caecal bacteria; Z. Y., M. O., M. T. and T. O. performed analysis of faecal organic acids; T. T., Y. I. and T. Y. analysed data and were also involved in editing the manuscript. All authors discussed the results and commented on the manuscript.
Y. N. received scholarship funds from Eisai Co. Ltd, Astellas Pharma Inc., Takeda Pharmaceutical Co. Ltd and Mitsubishi Tanabe Pharma Co. Ltd. The other authors have no conflicts of interest to disclose.










