The incidence of the metabolic syndrome characterised by insulin resistance, dyslipidaemia and hypertension is increasing worldwide. This is also associated with increased morbidity and mortality from several prevalent diseases, such as diabetes, cancer, myocardial infarction and stroke. Recent findings have shown that dietary fructose facilitates metabolic derangement and induces oxidative damage(Reference Levi and Werman1–Reference Basciano, Federico and Adeli5). Also, numerous studies suggest that increased fructose consumption may be an important contributor to the metabolic syndrome(Reference Hallfrisch, Reiser and Prather6–Reference Elliott, Keim and Stern12). In addition, a high-fructose diet leads to a well-characterised metabolic syndrome, typically resulting in hyperinsulinaemia, insulin resistance, hypertension, hypertriacylglycerolaemia, dyslipidaemia and a decline in the level of HDL-cholesterol(Reference Hwang, Ho and Hoffman13, Reference Reaven14). Also, high-fructose diet-fed animals have been shown to exhibit altered lipid metabolism due to hepatic stress as a result of the burden of fructose metabolism(Reference Kelley, Allan and Azhar3). Recently, functional foods which possess antioxidant activity have attracted attention as agents possibly reducing the risk of the metabolic syndrome induced by a high-fructose diet(Reference Dimo, Rakotonirina and Tan15–Reference Li, Douglas and Maiyoh21).
Emblica officinalis Gaertn., commonly known as amla, is a member of the small genus of Emblica (Euphorbiaceae). It grows in tropical and subtropical parts of China, India, Indonesia and the Malay Peninsula. It is an important dietary source of vitamin C, minerals and amino acids, and also contains phenolic compounds, tannins, phyllembelic acid, phyllemblin, rutin, curcuminoides and emblicol. All parts of the plant are used for medicinal purposes. Especially, the fruit has been used in Ayurveda as a potent rasayana(Reference Thakur22) and traditional medicine for the treatment of diarrhoea, jaundice and inflammation(Reference Deokar23). In addition, the pulp of the fruit is smeared on the head to alleviate headaches and dizziness(Reference Perry24). Recently, amla extract has been tested for various pharmacological activities. The fruit extract was reported to exhibit hypolipidaemic(Reference Anila and Vijayalakshmi25), antidiabetic(Reference Sabu and Kuttan26) and anti-inflammatory activities(Reference Asmawi, Kankaanranta and Moilanen27) and inhibit retroviruses such as HIV-1(Reference El-Mekkawy, Meselhy and Kusumoto28), tumour development(Reference Jose, Kuttan and Kuttan29) and gastric ulcer(Reference Bandyopadhyay, Pakrashi and Pakrashi30). Moreover, amla extract has been shown to exhibit antioxidant properties(Reference Bhattacharya, Chatterjee and Ghosal31, Reference Anila and Vijayalakshmi32), and it has been reported that the aqueous extract of amla is a potent inhibitor of lipid peroxide formation and a scavenger of hydroxyl and superoxide radicals in vitro (Reference Jose and Kuttan33). In a previous study, we demonstrated the antioxidative property of amla using Cu2+-induced oxidised human LDL(Reference Kim, Yokozawa and Kim34). Also, we showed that amla, especially an ethyl acetate (EtOAc) extract of amla (a polyphenol-rich fraction), attenuates age-related hyperlipidaemia and renal dysfunction by oxidative stress(Reference Yokozawa, Kim and Kim35, Reference Yokozawa, Kim and Kim36). On the basis of these studies, the present study was carried out to evaluate the effect of the polyphenol-rich fraction of amla on fructose-induced metabolic syndrome, and we also determined its related protein expression in a rat model.
Materials and methods
Preparation of ethyl acetate extract of amla
The EtOAc extract was prepared by extracting the air-dried amla fruit pieces in water–EtOAc (1:4) at room temperature for 24 h. The extract was evaporated under a reduced pressure followed by lyophilisation. The yield was 12 % from the dried fruit pieces.
Total polyphenol and vitamin C contents of ethyl acetate extract
The total polyphenol content of the EtOAc extract of amla was measured by employing a colorimetric method using gallic acid as a standard. The vitamin C content was measured using HPLC.
HPLC analysis of polyphenol components in ethyl acetate extract
An HPLC system (Waters Co., Milford, MA, USA) was used for the analysis of the polyphenol components of the EtOAc extract. Samples were analysed using the reverse-phase column C18 Cosmosil AR II (25 × 0·4 cm, particle size 5 μm; Nakalai Tesque Inc., Kyoto, Japan) using 50 mm-phosphoric acid (A) and CH3CN (B) as a solvent at a flow rate of 0·8 ml/min. The gradient used was 5 % B in A solvent to reach 30 % B during the first 39 min, and 75 % B in A solvent at 54 min. Chromatograms were detected at 280 nm UV.
Animals and treatment
All surgical and experimental procedures were performed in accordance with the recommendations found in the Guide for the Care and Use of Laboratory Animals (37) and approved by the Institutional Animal Care and Use Committee of the University of Toyama. Wistar male rats (Japan SLC Inc., Hamamatsu, Japan) were maintained with water and food ad libitum at a constant humidity and temperature, with a light–dark cycle of 12 h. After adaptation for 7 d, the rats (average weight 217 (se 6) g) were randomised into four groups composed of eight rats each: a normal diet group, a high-fructose diet-fed group, and two groups supplemented with a 65 % high-fructose diet and administered the EtOAc extract of amla at 10 or 20 mg/kg body weight (BW) per d via oral administration. The high-fructose diet (Table 1) was supplied for 2 weeks. During the experimental period, consumption of diet was kept at the same level (16 g/d per rat). Rats consumed the same amount of fructose regardless of the treatment, and no significant differences in BW or the daily intake of fructose were observed between treatment groups. At the end of the study, blood was collected and the serum was separated by centrifuging the blood at 1000 g for 15 min at 4°C. After collecting blood samples, liver and epididymal fat tissues were excised immediately, weighed, and frozen in liquid N2. All serum and tissue samples were stored at − 80°C until use for the determination of biochemical markers.
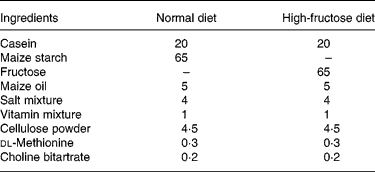
Table 1 Composition of the diets (g/100 g)
Blood sample preparation and analyses
The serum glucose and total cholesterol levels were determined using commercial reagents (Glucose CII-Test Wako and Cholesterol E-Test Wako, respectively; Wako Pure Chemical Industries, Ltd, Osaka, Japan). Lipoproteins were isolated from serum using density-gradient ultracentrifugation, as described by Havel et al. (Reference Havel, Eder and Bragdon38). Lipoprotein fractions were isolated from 4 ml serum using a Beckman Optima XL-70 ultracentrifuge and a 70·1 Ti rotor operating at 160 000 g. Serum was transferred to tubes, and the density was adjusted to 1·006, 1·019 and 1·063 g/ml with the same volume of KBr solution. Serum was divided into three lipoprotein classes by density: VLDL (d 1·006); intermediate-density lipoprotein (IDL; 1·006 < d < 1·019); LDL (1·019 < d < 1·603). The appropriate times were calculated to be 16 h for VLDL, 18 h for IDL and 20 h for LDL isolation at 4°C. TAG levels in serum and lipoprotein fractions were determined using a commercial reagent (Triglyceride E-Test Wako; Wako Pure Chemical Industries, Ltd, Osaka, Japan). Serum glycated protein and thiobarbituric acid (TBA)-reactive substance levels were measured using the methods of McFarland et al. (Reference McFarland, Catalano and Day39) and Naito & Yamanaka(Reference Naito and Yamanaka40), respectively.
Measurement of hepatic TAG and total cholesterol contents
The liver of each rat was homogenised, total lipids were extracted with a mixture of chloroform and methanol (2:1, v/v) according to the method of Folch et al. (Reference Folch, Lees and Sloane Stanley41), and the contents of TAG and total cholesterol were determined using the Wako kits described above.
Measurement of blood pressure
At the end of the experiment, blood pressure was measured by the tail-cuff method using an automatic blood pressure monitoring system (UR-5000; UETA, Tokyo, Japan). The animals were kept at 37°C for 30 min before measurements were performed. The average of five consecutive readings was used for blood pressure evaluation.
Isolation of hepatic mitochondria and measurement of thiobarbituric acid-reactive substance levels
The liver was homogenised with a nine-fold volume of ice-cold 0·9 % NaCl solution. Mitochondria were prepared from hepatic homogenates by differential centrifugation (800 g and 12 000 g; 4°C; 15 min) according to the methods of Johnson & Lardy(Reference Johnson and Lardy42) and Jung & Pergande(Reference Jung and Pergande43), respectively, with slight modifications. Each pellet was re-suspended in preparation medium, and the TBA-reactive substance concentration was determined by the method of Buege & Aust(Reference Buege and Aust44). Briefly, 250 μl of each re-suspended pellet or working standard was added to 750 μl of TBA–TCA–HCl solution (0·4 % of TBA, 15 % of TCA and 2·5 % HCl) and it was heated at 95–100°C for 20 min and cooled in an ice-bath. Then, samples were centrifuged at 1000 g at room temperature for 10 min to transfer supernatant fractions from the denatured protein precipitate. The TBA-reactive substance level was determined by measuring the absorbance at 532 nm. This was expressed in nmol malondialdehyde (MDA)/mg protein using a calibration curve constructed from MDA (0–25 nmol/ml) in 1,1,3,3-tetramethoxypropane. The protein level was evaluated by the method of Itzhaki & Gill(Reference Itzhaki and Gill45) with bovine serum albumin as the standard.
Homogenisation, isolation of cytosol and nuclear extracts
Each liver was homogenised by a Potter Elvehjem homogeniser in 4 volumes (w/v) of buffer A containing 25 mm-2-amino-2-hydroxymethyl-1,3-propanediol (Tris)-HCl (pH 7·5), 250 mm-NaCl, 5 mm-EDTA, 1 mm-phenylmethylsulfonyl fluoride, 1 mm-dithiothreitol and a mixture of protease inhibitors (100 mm-4-(2-aminoethyl)benzenesulfonyl fluoride, 0·08 mm-aprotinin, 2 mm-leupeptin, 5 mm-bestatin, 1 mm-pepstatin A and 1·5 mm-E-64). Homogenates were incubated for 15 min on ice, 10 % Nonidet P-40 was added, and then they were centrifuged at 4000 g at 4°C, for 5 min. Supernatant fractions were used for inducible NO synthase (iNOS), cyclo-oxygenase-2 (COX-2), bax and bcl-2 protein determination. Nuclear extracts were isolated using the method of Sakurai et al. (Reference Sakurai, Hisada and Ueno46). Briefly, liver was homogenised by a Potter Elvehjem homogeniser in 4 volumes (w/v) of buffer A containing 10 mm-HEPES (pH 7·9), 10 mm-KCl, 0·1 mm-EDTA, 1 mm-dithiothreitol, 0·5 mm-phenylmethylsulfonyl fluoride and protease inhibitors as above. Homogenates were incubated for 15 min on ice, 10 % Nonidet P-40 was added, and then they were centrifuged at 4000 g at 4°C for 5 min. Supernatant fractions were used for inhibitor binding protein κB-α (I-κBα) protein determination, and pellets were re-suspended in 2 volumes of buffer B containing 20 mm-HEPES (pH 7·9), 0·4 m-NaCl, 1 mm-EDTA, 1 mm-dithiothreitol, 1 mm-phenylmethylsulfonyl fluoride and the protease inhibitors. Homogenates were kept for 15 min at 4°C and then centrifuged at 14 000 g for 5 min at 4°C. Supernatant fractions were collected in microcentrifuge tubes, and used for PPARα, sterol regulatory element-binding protein (SREBP)-1/2 and NF-κB protein determination. The protein concentration of homogenates and nuclear extracts was determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Each sample (30 μg protein/lane) was denatured by boiling in Laemmli sample buffer and stored at − 80°C until the assay(Reference Laemmli47).
Western blot analyses
Homogenates (30 μg for iNOS, COX-2, bax and bcl-2), cytosol extract (30 μg for I-κBα) and crude nuclear extracts (30 μg for PPARα, SREBP-1/2 and NF-κB) from the liver were subjected to SDS-PAGE (10 %, w/v). The separated proteins were blotted onto nitrocellulose (Bio-Rad, Hercules, CA, USA). Blots were blocked overnight at 4°C with 5 % non-fat dry milk in TBS-T (25 mm-Tris-HCl (pH 8·3), 140 mm-NaCl, 2 mm-KCl and 0·1 % Tween 20). Membranes were then incubated for 3 h at 4°C with the primary polyclonal antibodies raised against NF-κB, I-κBα, bax, PPARα and SREBP-1/2 (dilution, 1:1000), and monoclonal antibodies against iNOS, COX-2, bcl-2 (dilution, 1:1000) and β-actin (1:5000) (antibodies from Santa Cruz Biotechnology, Santa Cruz, CA, USA). After extensive washing, incubation with the second antibody (rabbit polyclonal or mouse monoclonal antibody) at a dilution of 1:1000 (Santa Cruz Biotechnology) was also performed for 40 min at room temperature. Specific protein was detected by enhanced chemiluminescence (ECL; Amersham International, Little Chalfont, Bucks, UK) and quantified with a Phosphor Imager (Bio-Rad Laboratories, Hercules, CA, USA). Band densities were determined using ATTO Densitograph Software (ATTO Corporation, Tokyo, Japan) and quantified as the ratio to β-actin. These protein levels of groups were expressed relative to those of normal diet-fed rats.
Statistical analysis
The results are expressed as mean values with their standard errors. The effect on each parameter was examined using one-way ANOVA. Individual differences between groups were evaluated using Dunnett's test, and those at P < 0·05 were considered significant.
Results
Total polyphenol and vitamin C contents of ethyl acetate extract
The total polyphenol content of the EtOAc extract was 80·4 (se 4·3) %; however, vitamin C was not in the present extract.
Polyphenol components of ethyl acetate extract
Chromatograms of the EtOAc extract of amla are shown in Fig. 1. Major components were gallic acid (peak A), ellagic acid (peak H) and ellagitannins. Ellagitannins comprised three compounds: corilagin, geraniin and chebulagic acid (peaks E, F and G, respectively). Components of polyphenol compounds in the EtOAc extract were 5·847 % gallic acid, 1·187 % corilagin, 4·303 % geraniin, 5·547 % chebulagic acid and 1·603 % ellagic acid. Furthermore, several peaks besides the above components were detected; that is, mucic acid mono- and di-gallate, and the mixtures including their isomers (eluted before peak A) were as follows: mucic acid 1,4-lactone 3-O-gallate (peak B), mucic acid 1,4-lactone 2-O-gallate (peak C) and furosin (peak D), as reported by Zhang et al. (Reference Zhang, Tanaka and Yang48).

Fig. 1 HPLC analysis of polyphenol components in an ethyl acetate extract of amla (Emblica officinalis Gaertn.). Peaks: A, gallic acid; B, mucic acid 1,4-lactone 3-O-gallate; C, mucic acid 1,4-lactone 2-O-gallate; D, furosin; E, corilagin; F, geraniin; G, chebulagic acid; H, ellagic acid. mAU, milli arbitrary units.
Characteristics of experimental animals
As shown in Table 2, the BW of the high-fructose diet-fed control rats (241·0 (se 4·0) g) was higher than that of the normal diet-fed rats (231·8 (se 9·2) g), but this was slightly decreased by the oral administration of the EtOAc extract of amla. Compared with the normal diet-fed rats, the absolute and relative liver weights were significantly increased in the high fructose-fed control rats (P < 0·001). The EtOAc extract of amla suppressed the increase in the liver weight. The weight of the epididymal fat pads was significantly higher in the high fructose-fed control rats (P < 0·001) as compared with the normal diet-fed rats, while their weight in the EtOAc extract of amla-fed group significantly decreased compared with the high fructose-fed control rats. Daily fluid intake was not affected by the high-fructose diet. Also, the high-fructose diet consumed over 2 weeks significantly increased the levels of serum glucose (54·5 %; P < 0·001) and total cholesterol (34·6 %; P < 0·001). The level of serum glucose led to a tendency toward a decrease, and the level of serum total cholesterol significantly decreased through the administration of the EtOAc extract of amla (P < 0·01). In addition, TAG levels in the serum, and the VLDL, IDL and LDL fractions markedly increased with the high-fructose diet (P < 0·001). The elevated levels were significantly and dose-dependently lowered by the EtOAc extract of amla. Systolic blood pressure was significantly higher in the high fructose-fed rats than in the normal rats (127·8 (se 2·2) v. 114·0 (se 1·0) mmHg, respectively; P < 0·001) and was effectively controlled by the EtOAc extract of amla.
Table 2 Characteristics of experimental animals
(Mean values with their standard errors for eight animals per group)

EtOAc, ethyl acetate; BW, body weight; IDL, intermediate-density lipoprotein.
Mean value was significantly different from that of the normal diet-fed rats: * P < 0·05, ** P < 0·01, *** P < 0·001.
Mean value was significantly different from that of the high-fructose diet-fed control rats: † P < 0·05, †† P < 0·01, ††† P < 0·001.
‡ EtOAc extract of amla (Emblica officinalis Gaertn.).
Lipid contents and protein (PPARα and sterol regulatory element-binding protein-1/2) levels in the liver
As shown in Fig. 2, the hepatic TAG contents in the fructose-fed control rats increased by 1·8-fold compared with the normal rats (109·9 (se 11·4) v. 60·0 (se 5·2) mg/liver per 100 g BW, respectively; P < 0·001). The hepatic TAG contents were significantly lowered in the EtOAc extract of amla-fed rats at the oral doses of 10 and 20 mg/kg BW per d compared with the high fructose-fed control rats by 28·1 and 33·8 %, respectively (P < 0·001). Also, the hepatic total cholesterol level of the high fructose-fed control rats increased 1·5-fold compared with the normal rats. However, the administration of the EtOAc extract of amla at the doses of 10 and 20 mg significantly decreased the hepatic total cholesterol levels by 19·1 and 24·6 %, respectively (P < 0·001). Moreover, SREBP-1 protein levels in the high fructose-fed control rats significantly increased 1·3-fold compared with the normal rats (P < 0·001). The oral administration of the EtOAc extract of amla at the doses of 10 and 20 mg/kg BW per d significantly decreased the level of SREBP-1 protein in the nuclei of the liver by 12·4 and 16·3 % compared with the high fructose-fed control rats, respectively. There was no significant difference in the protein levels of PPARα and SREBP-2 in the nuclei of the liver between the experimental groups (Fig. 3).
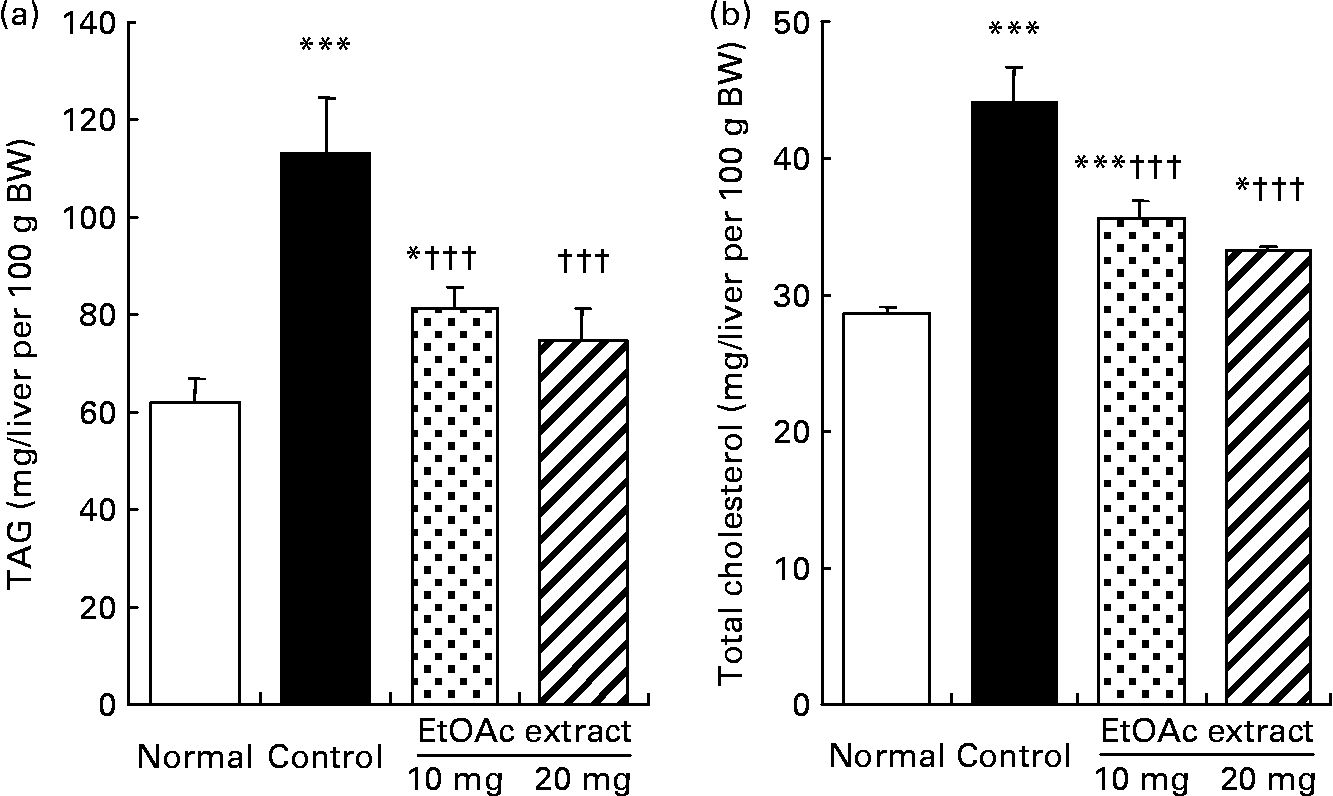
Fig. 2 Hepatic TAG (a) and total cholesterol (b) contents of rats fed a normal diet, a control high-fructose diet or a high-fructose diet supplemented with an ethyl acetate extract (EtOAc extract) of amla (Emblica officinalis Gaertn.), at 10 or 20 mg/kg body weight. Values are means for eight rats per group, with standard errors represented by vertical bars. Mean value was significantly different from that of the normal diet-fed rats: * P < 0·05, *** P < 0·001. ††† Mean value was significantly different from that of the high-fructose diet-fed control rats (P < 0·001).

Fig. 3 Western blot analysis of expressions of PPARα (a), sterol regulatory element-binding protein (SREBP)-1 (b) and SREBP-2 (c) in the liver of rats fed a normal diet, a control high-fructose diet or a high-fructose diet supplemented with an ethyl acetate extract (EtOAc extract) of amla (Emblica officinalis Gaertn.), at 10 or 20 mg/kg body weight. AU, arbitrary units. Values are means for eight rats per group, with standard errors represented by vertical bars. *** Mean value was significantly different from that of the normal diet-fed rats (P < 0·001). Mean value was significantly different from that of the high-fructose diet-fed control rats: † P < 0·05, †† P < 0·01.
Glycated protein and thiobarbituric acid-reactive substance levels in the serum and hepatic mitochondria
As shown in Fig. 4, the serum glycated protein level of the high fructose-fed control rats was higher than that of the normal diet-fed rats (38·3 (se 1·8) v. 34·5 (se 1·8) nmol/mg protein, respectively; P < 0·05). However, it was not affected by the EtOAc extract of amla. Furthermore, the TBA-reactive substance levels of serum were significantly higher in the high fructose-fed control rats than those in the normal diet-fed rats (4·1 (se 0·3) v. 1·9 (se 0·2) nmol/ml, respectively; P < 0·001). However, the administration of the EtOAc extract of amla at the dose of 20 mg/kg led to a significantly lower TBA-reactive substance level of serum, being 21·1 % lower than that in the fructose-fed control rats. Also, TBA-reactive substance levels of hepatic mitochondria were significantly lower in the EtOAc extract of amla-fed rats (43·1 %; P < 0·001) than in the fructose-fed control rats.

Fig. 4 Serum glycated protein (a), serum thiobarbituric acid (TBA)-reactive substance levels (b) and hepatic mitochondrial TBA-reactive substance levels (c) of rats fed a normal diet, a control high-fructose diet or a high-fructose diet supplemented with an ethyl acetate extract (EtOAc extract) of amla (Emblica officinalis Gaertn.), at 10 or 20 mg/kg body weight. Values are means for eight rats per group, with standard errors represented by vertical bars. Mean value was significantly different from that of the normal diet-fed rats: * P < 0·05, *** P < 0·001. Mean value was significantly different from that of the high-fructose diet-fed control rats: †† P < 0·01, ††† P < 0·001.
Protein levels involved in the pro-inflammatory state of the liver
Protein levels involved in the pro-inflammatory state of the liver in the high fructose-fed rats were examined by Western blot analysis (Fig. 5). The protein levels of hepatic NF-κB were significantly lower in the EtOAc extract of amla-fed rats than in the high fructose-fed control rats. However, there was no significant difference between the groups regarding the hepatic I-κBα and iNOS protein levels. The protein level of COX-2 in the liver was 24·4 and 26·0 % lower in the EtOAc extract of amla-fed rats than in the high fructose-fed control rats at the doses of 10 and 20 mg, respectively. Also, the bax protein level in the high-fructose diet-fed rats significantly increased by 18·0 % compared with the normal diet-fed rats (P < 0·01), whereas the EtOAc extract of amla-fed rats showed significant decreases of 8·5 and 10·2 % at the doses of 10 and 20 mg/kg BW per d, respectively. However, the bcl-2 protein level in the high fructose-fed rats significantly decreased by 21·0 % compared with the normal diet-fed rats (P < 0·001), while the oral administration of the EtOAc extract of amla led to significant increases by 19·0 and 24·1 % at the doses of 10 and 20 mg/kg BW per d, respectively, compared with the fructose-fed control rats.

Fig. 5 Western blot (a) analysis of protein expressions involved in the inflammatory status of the liver: NF-κB (b); inhibitor binding protein κB-α (I-κBα) (c); inducible NO synthase (iNOS) (d); cyclo-oxygenase-2 (COX-2) (e); Bcl-2 (f); Bax (g). Rats were fed a normal diet, a control high-fructose diet or a high-fructose diet supplemented with an ethyl acetate extract (EtOAc extract) of amla (Emblica officinalis Gaertn.), at 10 or 20 mg/kg body weight. AU, arbitrary units. Values are means for eight rats per group, with standard errors represented by vertical bars. Mean value was significantly different from that of the normal diet-fed rats: * P < 0·05, ** P < 0·01, *** P < 0·001. Mean value was significantly different from that of the high-fructose diet-fed control rats: † P < 0·05, †† P < 0·01, ††† P < 0·001.
Discussion
High-fructose diets have been used in animal models to induce the metabolic syndrome, including abdominal obesity, dyslipidaemia, hypertension, insulin resistance, microalbuminuria, and prothrombotic and pro-inflammatory states(Reference Ackerman, Oron-Herman and Grozovski4, Reference Basciano, Federico and Adeli5, Reference Reaven14, Reference Tobey, Mondon and Zavaroni49–Reference Fried and Rao52). In addition, high fructose-fed animals exhibit an alternation in lipid metabolism due to hepatic oxidative stress as a result of the burden of fructose metabolism(Reference Kelley, Allan and Azhar3). Our present study also showed that a high-fructose diet during 2 weeks induced metabolic alterations such as hyperglycaemia, dyslipidaemia and hypertension. Moreover, the present results showed that a high-fructose diet led to a significant increase in TAG levels in the serum and lipoprotein fractions. These results indicate that a low-fructose diet would play a role in ameliorating pathological conditions such as diabetes and CVD. Therefore, the rat model with fructose-induced metabolic syndrome was used in the present study to investigate the protective role of the polyphenol-rich extract of amla (EtOAc extract of amla) against the metabolic syndrome.
The EtOAc extract of amla significantly attenuated the increase in liver weight by the high-fructose diet and significantly decreased the weight of epididymal fat pads increased by the diet. Furthermore, the high-fructose diet elevated the serum glucose levels, which may indicate the progression of insulin resistance. The results suggest that the EtOAc extract of amla would probably play a protective role against the abnormal metabolism of carbohydrate induced by a high-fructose diet. In addition, hypertriacylglycerolaemia and TAG-rich lipoproteins are part of a metabolic syndrome frequently encountered in individuals with early-onset CHD(Reference Sarti and Gallagher53). VLDL, the main carrier of TAG, has a well-established indirect atherogenic potency as the precursor of LDL, and may promote the development of atherosclerotic lesions through activation of the pro-inflammatory transcription factor NF-κB(Reference Dichtl, Nilsson and Goncalves54–Reference Williams, Maitin and Jackson56). However, the EtOAc extract of amla reduced TAG levels that had markedly increased in the serum, VLDL, IDL and LDL fractions by the high-fructose diet. These findings imply that the EtOAc extract of amla may reduce the development of atherosclerotic lesions by inhibiting the oxidative modification of VLDL and LDL in the arterial wall.
Moreover, the present results showed that the oral administration of the EtOAc extract of amla ameliorated the increase of hepatic TAG content with the regulation of blood pressure. These results indicate that the EtOAc extract of amla would protect against hypertriacylglycerolaemia and hypertension induced by a high-fructose diet. In addition, it inhibited the increase of total cholesterol level in the liver. Our previous study also demonstrated that amla prevented hypercholesterolaemic atherosclerosis and attenuated the risk of CVD by not only reductions in LDL-cholesterol and its oxidation, but also the decline in lipid peroxidation(Reference Kim, Yokozawa and Kim34). Based on this evidence, the polyphenol-rich fraction of amla is expected to play a protective role against the metabolic syndrome related to a high-fructose diet including hypertriacylglycerolaemia, dyslipidaemia and hypertension.
The β-oxidation of fatty acids and the synthesis of fatty acids and TAG in the liver are regulated by the nuclear receptors PPARα and SREBP-1, respectively(Reference Kersten, Desvergne and Wahli57, Reference Roglans, Sanguino and Peris58). In addition, SREBP-2 preferentially activates cholesterol synthesis. PPARα plays an important role in the metabolic homeostasis of fatty acids through the regulation of target genes encoding enzymes for fatty acid β-oxidation and fatty acid transporters(Reference Schoonjans, Martin and Staels59–Reference Leone, Weinheimer and Kelly62). The rats fed a high-fructose diet and the EtOAc extract of amla did not show an altered expression of PPARα, suggesting no significant role in fatty acid β-oxidation. On the other hand, the hepatic SREBP-1 protein level was increased, without changes in PPARα and SREBP-2, resulting in the elevation of serum and hepatic TAG levels by the high-fructose diet. Miyazaki et al. (Reference Miyazaki, Dobrzyn and Man63) also reported the induction of hepatic mRNA and protein levels of SREBP-1 and lipogenic gene expression including fatty acid synthase, acetyl-CoA carboxylase and stearoyl-CoA desaturase, whereas SREBP-2 proteins remained unchanged in mice following 7 d on a 60 % fructose diet. However, the EtOAc extract of amla resulted in the suppression of the hepatic SREBP-1 protein level, which probably plays a crucial role in decreasing the hepatic TAG contents. These results suggest the possibility that the EtOAc extract of amla would lower the serum and hepatic TAG levels through a signalling pathway that regulates TAG synthesis but not the β-oxidation of fatty acids. On the other hand, although the hepatic total cholesterol content on high fructose feeding was significantly increased, the protein level of SREBP-2, a key transcription factor controlling cholesterol biosynthesis, was not affected by the high fructose feeding or administration of the EtOAc extract of amla. It is thought that the reduction of the hepatic total cholesterol content caused by the EtOAc extract of amla is not associated with hepatic cholesterol synthesis, but probably involved in other mechanisms such as cholesterol excretion.
Recent studies have shown that the metabolic syndrome is associated with the generation of reactive oxygen species (ROS) and reduction of certain antioxidants(Reference Aljada, Garg and Ghanim64–Reference Dandona, Aljada and Chaudhuri70). In addition, Delbosc et al. (Reference Delbosc, Paizanis and Magous71) also reported that high fructose feeding is associated with an early (1 week) increase in ROS production by aorta, heart and circulatory polymorphonuclear cells, in association with enhanced markers of oxidative stress. Therefore, oxidative stress induced by a high-fructose diet is attributed to the metabolic syndrome, since a high-fructose diet alters lipid metabolism and dysregulation in the liver. The present results show that TBA-reactive substance levels in the serum and hepatic mitochondria were increased in rats fed a high-fructose diet. However, the EtOAc extract of amla reduced the elevated TBA-reactive substance levels in serum and hepatic mitochondria. Our previous study also provides supporting evidence that amla exhibits an antioxidative activity and protective effect against oxidative stress(Reference Kim, Yokozawa and Kim34). These results imply that the EtOAc extract of amla may ameliorate high fructose-induced metabolic syndrome by reducing oxidative stress, and this effect may be due to the antioxidant effect of the EtOAc extract of amla which contains polyphenols.
High levels of inflammation increase the risk of developing diabetes and atherosclerosis, and are thought to be a possible mechanism for the adverse consequences of the metabolic syndrome(Reference Barzilay, Abraham and Heckbert72, Reference Pradhan, Manson and Rifai73). Therefore, we determined the effect of an EtOAc extract of amla on inflammatory protein levels caused by oxidative stress in the liver in a rat model with a high fructose-induced metabolic syndrome. Under resting conditions, NF-κB exists in the cytoplasm as a dimer bound to the inhibitory protein I-κBα. Inducers of NF-κB, such as inflammatory cytokines, ROS and viral products, activate a dimeric I-κBα kinase complex, causing the phosphorylation and ubiquitination of I-κBα and its release from NF-κB. The free NF-κB dimer translocates to the nucleus, where it regulates target gene transcription such as iNOS, COX-2, IL-6, IL-12 and TNF-α(Reference Baldwin74, Reference Li and Verma75). The activation of NF-κB activity, in turn, up-regulates the synthesis of anti-apoptotic members, the bcl-2 family(Reference Sasaki, Kumazaki and Takano76), and increases the transcription of genes that encode protective enzymes such as iNOS and COX-2(Reference Chung, Kim and Kim77). Our data showed that the EtOAc extract of amla attenuated the increase of hepatic COX-2 protein by the fructose diet through regulation of the NF-κB signalling pathway. However, the EtOAc extract of amla did not alter the expressions of I-κBα and iNOS, indicating that there might be another mechanism regulating the I-κBα and iNOS protein level. Moreover, in our present results, the bax protein level was significantly enhanced in the high fructose-induced metabolic syndrome rat model, while bcl-2 protein was significantly reduced compared with the normal rats. The oral administration of the EtOAc extract of amla has a beneficial effect on these proteins. These results suggest that the EtOAc extract of amla may reduce the severity of hepatic inflammation and liver cell injury induced by a high-fructose diet on regulating the related protein expression. It has been demonstrated that the disruption of the NF-κB pathway under hypertriacylglycerolaemic and hyperglycaemic stress responses inhibits oxidative stress and inflammatory responses(Reference Kelley, Allan and Azhar3, Reference Dichtl, Nilsson and Goncalves54, Reference Stentz, Umpierrez and Cuervo69). These findings indicate that the protective potential of the EtOAc extract of amla against the metabolic syndrome is attributed to the regulation of COX-2, NF-κB, bcl-2 and bax signalling pathways, and can be explained by its antioxidant effect derived from its polyphenolic constituents.
From the present study, we conclude that EtOAc extract of amla would improve high-fructose diet-induced metabolic syndrome, including hyperglycaemia, hyperlipidaemia and hypertension. The administration of the EtOAc extract of amla ameliorated the metabolic syndrome through the reduction of TAG and cholesterol concentrations, with the regulation of the hepatic SREBP-1 protein level and the suppression of inflammation by regulating the COX-2 and NF-κB protein levels. Further studies to identify active components in the polyphenol-rich fraction of amla should be conducted to elucidate the mechanism of its protective effect against the metabolic syndrome.
Acknowledgements
The present study was supported in part by Grants-in-Aid (C) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (no. 19500661 to T. Y.).
H. Y. K., T. O. and L. R. J. conducted the experimental work. T. Y. designed the experiment and wrote the manuscript.
The authors state that there are no conflicts of interest.











