People with chronic kidney disease (CKD) are at increased risk of mortality and CVD( Reference Go, Chertow and Fan 1 , Reference Matsushita and van der Velde 2 ), even with relatively small decreases in glomerular filtration rate <60 ml/min per 1·73 m2 ( Reference Matsushita and van der Velde 2 , Reference Vanholder, Massi and Argiles 3 ). It is well-known that control of blood pressure (BP) and proteinuria are pivotal for the preservation of renal function and prevention of complications associated with CKD( Reference Lambers Heerspink, de Borst and Bakker 4 ).
There is growing evidence that dietary sodium restriction improves BP control in the general population, and also in those with CKD( Reference Campbell, Johnson and Bauer 5 , Reference He, Li and Macgregor 6 ). Recent randomised controlled trials showed that dietary sodium restriction significantly decreased BP( Reference de Brito-Ashurst, Perry and Sanders 7 , Reference McMahon, Bauer and Hawley 8 ), and consistent reductions in proteinuria were also observed in these groups of patients, independent of BP changes( Reference McMahon, Bauer and Hawley 8 ). Furthermore, avoidance of excessive dietary sodium intake has been shown to enhance the effect of single-agent renin–angiotensin–aldosterone system blockade to improve renal and cardiovascular outcomes in two post hoc analyses of clinical trials( Reference Vegter, Perna and Postma 9 , Reference Lambers Heerspink, Holtkamp and Parving 10 ). In a recently published systematic review that explored the association between sodium intake and renal outcomes, the authors concluded that the available, but limited, evidence supports an association between high sodium intake (>4·6 g/d) and adverse outcomes. However, the association with low (<2·3 g/d) v. moderate (2·3–4·6 g/d) sodium intake is uncertain, with inconsistent findings from cohort studies( Reference Smyth, O’Donnell and Yusuf 11 ). In this study, the authors concluded that these data support reducing dietary sodium intake in CKD, but additional research is required to determine the optimum target sodium intake( Reference Smyth, O’Donnell and Yusuf 11 ). Furthermore, most of the published data relate to people managed in secondary care with more severe or advanced CKD, and it is therefore not clear whether these findings are relevant to people managed in primary care with less severe or early stage CKD.
At present, most guidelines for the management of CKD recommend that sodium intake should be restricted to <2·3–2·4 g/d (100 mmol/d, equivalent to 6 g/d of salt)( 12 – Reference Levin, Hemmelgarn and Culleton 14 ), although the few studies available in people with CKD indicate that the majority of people do not adhere to this recommendation( Reference Yu, Luying and Haiyan 15 – Reference Nerbass, Pecoits-Filho and McIntyre 18 ).
We have previously investigated sodium intake in a large cohort of people with CKD stage 3 managed in primary care( Reference Nerbass, Pecoits-Filho and McIntyre 18 ) and found that at baseline excessive sodium intake was an independent determinant of mean arterial pressure (MAP) and albuminuria( Reference Nerbass, Pecoits-Filho and McIntyre 19 ). In the present study, we further investigated the role of sodium intake by examining the effect of changes in sodium intake over 1 year on BP, pulse wave velocity (PWV) and proteinuria.
Methods
Participants and recruitment
Participants were recruited from a prospective cohort of people with CKD stage 3 in primary care, the Renal Risk in Derby (RRID) study. The methods for the RRID study have been published in detail elsewhere( Reference McIntyre, Fluck and McIntyre 20 ). In summary, eligible participants were aged 18 years or over, met the Kidney Disease Outcomes Quality Initiative criteria for CKD stage 3 (estimated glomerular filtration rate (eGFR) of 30–59 ml/min per 1·73 m2 on two or more occasions at least 3 months apart before recruitment), were able to give informed consent and were able to attend their general practitioner (GP) surgery for assessments. People who had previously had a solid organ transplant or who were terminally ill (expected survival <1 year) were excluded. The RRID study is conducted by a single nephrology department, but participants were recruited directly from thirty-two GP surgeries.
Data collection
The first study visits were conducted from August 2008 to March 2010. Screening and baseline visits were combined due to the large proportion of elderly participants and the logistical challenges associated with conducting study visits in multiple primary-care centres. Participants were sent a medical and dietary questionnaire, as well as three urine specimen bottles, and were asked not to eat cooked meat for at least 12 h before the assessment. Urine was collected as three early morning samples. Socio-economic status was defined using the Indices of Multiple Deprivation score and the self-reported educational status. At the assessment, information on questionnaires was checked, anthropomorphic measurements were taken and urinalysis was performed. Blood specimens were taken and the three urine specimens were submitted for biochemical analysis. eGFR was calculated using the modified four-variable Modification of Diet in Renal Disease equation.
BP was measured after a minimum of 5 min rest in the sitting position, using a validated oscillometric device, recommended by the British Hypertension Society (Digital Blood Pressure Monitor Model UA-767; A&D Instruments Ltd). The same device was used for all readings. BP was calculated as the mean of three readings that differed by <10 %. The MAP was calculated as 1/3 the average systolic BP (SBP) plus 2/3 the average diastolic BP (DBP).
Albuminuria was assessed by measuring the urinary albumin:creatinine ratio (UACR) on three consecutive early morning urine specimens collected before the clinic visit and stored in a refrigerator. The average of the three values was used for the analysis and patients with UACR>3 mg/mmol were considered albuminuric( 21 ).
Serum high-sensitivity C-reactive protein (hsCRP™; Roche Diagnostics) was measured using a Roche Modular P Analyser (Roche Diagnostics) run in accordance with the manufacturer’s instructions.
Carotid to femoral PWV was measured as a marker of arterial stiffness (AS). Measurements were performed using a VicorderTM device (Skidmore Medical Ltd) and were carried out in the semiprone position (at approximately 30°) to prevent venous contamination of the arterial signal.
Using the coefficients from a regression equation, we previously developed the following formula to estimate 24 h urinary sodium excretion (24 hUNa) from weight and early morning urinary sodium concentration (EM UNa)( Reference Nerbass, Pecoits-Filho and McIntyre 22 ):
Although the accuracy of the formula was low, its ability to discriminate between sodium excretion above or below the KDOQI guideline (≤100 mmol/d, corresponding to 6 g NaCl/d) was better, with good sensitivity (85 %), positive predicted value of 70 % and negative predicted value of 70 %. The EM UNa value used to estimate 24 hUNa was the average of measurements on morning urine specimens collected on 3 consecutive days. Sodium intake was assumed to be equal to 24 hUNa. BMI was calculated from weight in kilograms divided by height squared in metres and categorised according to WHO categories( 23 ). Diabetes was defined as having a previous clinical diagnosis in line with WHO criteria( 24 ). Previous cardiovascular event (CVE) was defined as subject-reported myocardial infarction, stroke, transient ischaemic attack, re-vascularisation or amputation due to peripheral vascular disease or aortic aneurysm. Smoking status was categorised as never smoked, ex-smoker and current smoker. Self-reported alcohol consumption was categorised as never or ever drinking, irrespective of the kind or quantity. All baseline assessments were repeated after 1 year from 2009 to 2011.
The study was approved by the Nottingham Research Ethics Committee 1. All the participants provided written informed consent. The study was included in the National Institute for Health Research (NIHR) Clinical Research Portfolio (NIHR Study ID: 6632) and was independently audited by QED Clinical Services in November 2009.
Statistical analysis
For statistical analysis, participants were assigned to three groups according to changes in sodium intake status between baseline and year-1 assessments: unchanged, increased (from ≤100 to >100 mmol/d) and decreased (from >100 to ≤100 mmol/d). Continuous variables are reported as the mean values and standard deviations when normally distributed or as the median and interquartile range when not. One-way ANOVA and t test were used to compare groups where variables were normally distributed and the Kruskal–Wallis and the Mann–Whitney U test were used if not. Paired samples t test and Wilcoxon signed rank test were used to compare changes in the same subjects over baseline and 1-year follow-up according to the distribution of variables.
Variables with skewed distribution (exponential) were log-transformed for analysis. Univariable linear regression analysis was used to evaluate associations between change in sodium intake and MAP or UACR. Multivariable linear regression analysis, using the forward stepwise method, was used to identify independent determinants of MAP or UACR. P<0·05 was used for a variable to enter the model. The adjusted R 2 value is reported as a measure of goodness of fit. The regression coefficients and their 95 % confidence intervals and standardised coefficients and their β coefficients from the final multivariable model are presented.
IBM SPSS Statistics for Windows version 21 was used for the analysis.
Results
A total of thirty-eight participants died before year-1 follow-up visits, forty-three withdrew or were lost to follow-up and data were incomplete in a further fifty-three. Thus, only 1607 of the original 1741 participants were included in this analysis.
Baseline characteristics for the entire RRID study population and three sub-groups defined by change in sodium intake over 1 year are presented in Table 1. There were more women (60·6 %) than men, and most participants were aged 65 years or above (81·8 %) The mean estimated sodium intake was 113 (sd 34) mmol/d.
Table 1 Main baseline characteristics of the whole population and the three groups defined by changes in sodium intake (Mean values and standard deviations; median values and interquartile ranges)

eGFR, estimated glomerular filtration rate; DM, diabetes mellitus; UACR, urinary albumin:creatinine ratio; SBP, systolic blood pressure; DPB, diastolic blood pressure; MAP, mean arterial pressure; PWV, pulse wave velocity; hsCRP, high-sensitive C-reactive protein.
* P<0·05 for trend; ** P<0·05 for increased v. decreased.
After 1 year, the mean estimated sodium intake was 112 (sd 34) mmol/d. We observed that 88 % of the people remained in the same category of sodium intake after 1 year, 32·4 % in the recommended sodium intake category (≤100 mmol/d) and 55·6 % in the high sodium intake category (>100 mmol/d). We also found that 6.5 % decreased their intake from >100 mmol/d at baseline to ≤100 mmol/d and 5·4 % increased their intake.
By comparing the three groups defined by change in sodium intake, there were significant differences in weight, BMI, SBP and sodium intake at baseline (Table 1). People who decreased their sodium intake evidenced a greater proportion of males as well as higher weight, SBP and sodium intake at baseline than those who increased their sodium intake (Table 1).
Changes in sodium intake and several risk factors over 1 year are shown in Table 2. People who decreased their sodium intake also evidenced decreases in eGFR, weight, SBP, DBP, MAP and PWV. Changes in MAP among the three groups are also shown in Fig. 1. There were no associations between demographic variables and change in sodium intake (data not shown).

Fig. 1. Changes in mean arterial pressure (MAP) over 1 year among the three groups defined according to changes in sodium intake.
Table 2 Changes in sodium intake and risk factors over 1 year among groups defined by changes in sodium intake (decreased, unchanged and increased) (Mean values and standard deviations; median values and interquartile ranges)

eGFR, estimated glomerular filtration rate; UACR, urinary albumin:creatinine ratio; SBP, systolic blood pressure; DPB, diastolic blood pressure; MAP, mean arterial pressure; PWV, pulse wave velocity.
* P<0·05 for increased v. decreased; ** P<0·001 for increased v. decreased.
A sub-group analysis including only participants with albuminuria at baseline found that albuminuria decreased only in those who decreased their sodium intake (n 20; UACR decreased from 7·7 (4·1–41·2) to 5·1 (3·3–15·9) mg/mmol; P=0·003).
Table 3 presents baseline and year-1 data of people with low sodium intake at both time points v. those with high sodium intake at both time points. BP improved in both groups, but the number of anti-hypertensive increased only in the higher sodium intake group, and PWV improved only in participants with lower sodium intake. Weight decreased slightly in the low sodium intake group, and albuminuria increased in both the sub-groups.
Table 3 Comparison of risk factors variables between baseline and year 1 in patients who remained in the lower or higher sodium intake groups (Mean values and standard deviations; median values and interquartile ranges)

eGFR, estimated glomerular filtration rate; UACR, urinary albumin:creatinine ratio; SBP, systolic blood pressure; DPB, diastolic blood pressure; MAP, mean arterial pressure; PWV, pulse wave velocity.
Univariate analysis to assess determinants of ΔMAP over 1 year in the whole population identified Δweight, ΔeGFR, Δnumber of anti-hypertensives, alcohol consumption status, diabetes mellitus status and decrease in estimated sodium intake from >100 to ≤100 mmol/d. In the multivariable linear regression analysis, which included all these variables, the model identified all the above-mentioned variables as independent determinants of ΔMAP (Table 4). A change in sodium intake status from high (>100 mmol/d) to low (≤100 mmol/d) was associated with a corrected decrease in MAP of 3·43 mmHg, an effect size similar to that observed with an increase by one in the number of anti-hypertensives.
Table 4 Independent determinants of Δmean arterial pressure (ΔMAP) (Regression coefficients, 95 % confidence intervals and β coefficients)
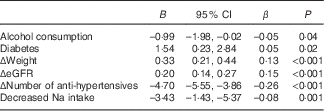
eGFR, estimated glomerular filtration rate.
R 2=0·13.
Discussion
High sodium intake has been implicated in CVD and CKD progression. The present study investigated the role of sodium intake by examining the effect of changes in sodium intake over 1 year on BP and proteinuria in people with early stage CKD. We found that people with CKD who decreased their sodium intake evidenced a decrease in all BP variables as well as PWV v. those who increased their intake. Furthermore, in people who maintained a low sodium intake, we observed a decline in both BP and PWV over 1 year, whereas people who maintained a high sodium intake showed a decrease in BP but not in PWV. Decrease in sodium intake was an independent determinant of ΔMAP but not ΔUACR. However, in a sub-group analysis that included only albuminuric participants, there was an improvement in albuminuria only in people who decreased their sodium intake.
Sodium intake, blood pressure and pulse wave velocity
The significant decline in BP observed in our participants who decreased their sodium intake (11/6 mmHg) was similar to the results obtained by interventional studies in people with CKD. The LowSALT CKD study – a 6-week double-blind, placebo-controlled, randomised cross-over study in twenty adult patients with hypertensive stages 3–4 CKD – demonstrated that with a reduction in 24 h sodium excretion from 168 to 75 mmol/d, 24-h ambulatory BP decreased by 10/4 mmHg( Reference McMahon, Bauer and Hawley 8 ). In another randomised controlled study performed in a population with very high sodium intake at baseline, urinary sodium excretion dropped from 260 to 103 mmol/d at 6 months in the intervention group and resulted in mean reductions in 24 h SBP/DBP of 8/2 mmHg( Reference de Brito-Ashurst, Perry and Sanders 7 ). Finally, in a 7-d intervention study with twenty Chinese participants, a BP decrease of 11/4 mmHg was achieved with a change in 24-h sodium excretion from 134 to 96 mmol/d( Reference Yu, Luying and Haiyan 15 ). All these considerable reductions in BP are comparable with that expected from the addition of a further anti-hypertensive medication( Reference Heran, Wong and Heran 25 ). In fact, efforts to reduce dietary sodium intake are particularly effective in this population( Reference Luther 26 ), as people with CKD are generally considered to represent a salt-sensitive population due to the inability to excrete a sodium load and diminished sodium buffering capacity( Reference Koomans, Roos and Boer 27 ). Multivariable analysis confirmed that a decrease in sodium intake was a determinant of ΔMAP, independent of other significant determinants including diabetes, alcohol intake and changes in number of anti-hypertensives, eGFR or body weight.
We observed that participants who decreased their sodium intake also had an improvement in PWV, a marker of AS that has been identified as a non-traditional risk factor associated with the large cardiovascular risk burden in CKD( Reference Safar, London and Plante 28 , Reference Mitchell 29 ). Based on previously published data from participants with CKD stages 3–5, the observed mean difference in PWV of 0·55 m/s at year 1 between those who decreased v. those who increased their sodium intake (Table 2) could be estimated to be associated with a 8 % decrease in all-cause mortality and a 6 % decrease in CVE. Similarly, the 0·25 m/s mean difference in PWV between those who maintained a low v. those who maintained a high sodium intake (Table 3) could be estimated to be associated with a 4 % decrease in all-cause mortality and a 3 % decrease in CVE( Reference Karras, Haymann and Bozec 30 ). AS in CKD is proposed to provoke an increase in SBP and pulse pressure (PP). This in turn leads to an increase in ventricular afterload, myocyte hypertrophy and reduced coronary perfusion, resulting in systolic and diastolic dysfunction. Elevated systolic and PP may also contribute to vascular damage, further increasing cardiovascular risk( Reference Chue, Townend and Steeds 31 ). Change in PWV was not observed in the LowSALT CKD study probably due to the short duration of follow-up( Reference McMahon, Bauer and Hawley 8 ).
The small but significant difference observed in changes in eGFR between patients who decreased and patients who increased their sodium intake is consistent with observations in a cross-over intervention study, in which a high sodium intake resulted in an increase of 30 % in eGFR( Reference McMahon, Bauer and Hawley 8 ). Similarly, other studies that have shown that a high sodium intake can result in increased creatinine clearance( Reference Suckling, He and Macgregor 32 ) due to glomerular hyperfiltration associated with increased intra-glomerular pressure( Reference Luik, Hoogenberg and Van Der Kleij 33 , Reference Mallamaci, Leonardis and Bellizzi 34 ).
Our results also show that, besides decreasing sodium intake, maintaining it below recommended levels was associated with improved BP control and decreased PWV without changes in the number of anti-hypertensives. However, in the high sodium intake group, better BP control was also observed, but PWV did not improve and the number of anti-hypertensive increased over 1 year.
Sodium intake and albuminuria
Although decreasing sodium intake was not an independent determinant of changes in albuminuria (data not shown), participants in a small sub-group who had albuminuria at baseline and who decreased their intake evidenced an improvement in their UACR. This is important because proteinuria is an important risk factor for CKD progression, and reduction of proteinuria is a key component of strategies for achieving renal and cardiovascular protection( Reference Lambers Heerspink, de Borst and Bakker 4 ). Our data, although in a small sub-group, are consistent with recent analyses reporting interactions between the impact of dietary sodium intake and proteinuria( Reference Fan, Tighiouart and Levey 35 , Reference McQuarrie, Traynor and Taylor 36 ).
The relationship between sodium intake and proteinuria appears to be even more robust in studies performed in secondary care. In the LowSALT CKD study, urinary protein-to-creatinine ratio and UACR were significantly reduced on a low-sodium diet compared with a high-sodium diet, independent of BP control( Reference McMahon, Bauer and Hawley 8 ). A decrease of 465 mg/d in urine protein excretion was also achieved after a decrease in sodium intake of 40 mmol/d in a Chinese 7-d intervention study( Reference Yu, Luying and Haiyan 15 ). Of particular importance, several studies have consistently demonstrated that dietary sodium restriction enhances the BP and albuminuria response to angiotensin-receptor blockers in both diabetic and non-diabetic patients with CKD( Reference Luther 26 , Reference Ekinci, Thomas and Thomas 37 ).
Limitations and strengths of the study
Our study has some limitations. First, we did not use a gold standard method to estimate sodium intake (24 h sodium excretion) due to the large number of participants and high proportion of older people. Nevertheless, the formula used to estimate sodium intake from early morning urine samples was specially developed for this study population, and we have previously reported that the method used has a good sensitivity for identifying people with high estimated sodium intake( Reference Nerbass, Pecoits-Filho and McIntyre 22 ). Second, the change in sodium intake was based on only two evaluations, although each used the average of three consecutive early morning urine specimens and the associations observed were in agreement with the results reported in better controlled studies. Third, it is possible that those people who reduced their sodium intake also adopted other lifestyle measures that may have had a beneficial effect on their BP – for example, weight loss and exercise. Thus, it is not possible to attribute all of the benefit of BP control to dietary sodium restriction alone, but an independent effect is supported by our finding that the association between reduction in dietary sodium and change in MAP was independent of change in weight. Fourth, data regarding anti-hypertensive use were not comprehensive because we lacked information on dose changes. Thus, it is possible that some of the improvement in BP observed may have been due to changes in the dose of anti-hypertensives. Finally, although our participants are representative of people with CKD cared for by primary-care centres in the UK, the majority of them were of Caucasian ethnicity, and thus our findings may not be directly applicable to other populations. Strengths of the study include the large cohort size, standardisation of BP and other measures as well as the use of three morning urine samples to assess sodium levels and proteinuria.
Conclusion
In this large prospective cohort study, we have found that people with relatively early stage CKD followed-up in primary-care centres, who decreased their sodium intake to <100 mmol/d over 1 year, had a decrease in all BP variables as well as PWV v. those who increased their sodium intake, and albuminuric participants who decreased sodium intake improved their albuminuria. In addition, in people who maintained a low sodium intake over this period, we observed a decline in both BP and PWV over 1 year, whereas people who maintained a high sodium intake showed a decrease in BP associated with an increase in the number of anti-hypertensive and no improvement in PWV. Furthermore, a decrease in sodium intake was an independent determinant of ΔMAP. Although further evidence is needed, our results support the benefits of reducing and maintaining sodium intake below 2·3–2·4 g/d (100 mmol) in people with early stage CKD.
Acknowledgements
This collaboration was facilitated by the Sister Renal Centers Program of the International Society of Nephrology.
This study was supported by a fellowship and grants from Kidney Research UK and British Renal Society (awarded to N. J. M.) and the CAPES Foundation, Ministry of Education of Brazil (awarded to F. N.), as well as an unrestricted educational grant from Roche Products PLC. Roche Products had no role in the design, analysis or writing of this article.
The authors’ contributions are as follows: F. B. N.: statistical analysis and interpretation of data and drafting of the manuscript. R. P.-F.: interpretation of data, critical revision and supervision. N. J. M.: conception and design, acquisition of data, obtaining funding and approved final version. A. S.: acquisition of data, analysis of data and approved final version. C. W. M.: conception and design, obtaining funding and critical revision. M. W. T.: conception and design, obtaining funding, statistical analysis and interpretation of data, critical revision and supervision.
The authors declare that they have no conflicts of interest.










