Neurological manifestations associated with Crohn’s disease (CD), which are probably underdiagnosed, may lead to perform a brain magnetic resonance imaging (MRI).Reference Kosmidou, Katsanos and Katsanos1 Identification of white matter hyperintensities (WMHs) in a patient with CD is not uncommon and can result from different mechanisms that can sometimes coexist. In this letter, we aim at discussing the different possible etiologies of WMH in a patient with an initial diagnosis of CD treated with anti-tumor necrosis factor (TNF)-α infliximab, which finally proved to be mitochondrial neurogastrointestinal encephalopathy (MNGIE) with a CD-like presentation.
A 46-year-old female was referred to a gastroenterologist because of weight loss over the last 3 months (body mass index fell from 18.2 to 14.2 kg/m2) in a context of chronic abdominal pain since the age of 25, and vomiting in her late 40s. Her sister was considered to have suffered from CD and died at the age of 50 from severe undernutrition. Her parents were healthy.
The colonoscopy was considered normal, but magnetic resonance enterography found suspected segmental inflammatory lesions and stenosis, respectively, in the distal and proximal small bowel, which were consistent with CD.
After the failure of oral corticosteroids, anti-TNF-α infliximab was started at the age of 47. Four months later, she complained about a loss of pain and heat sensations in both hands and balance problems. A brain MRI was performed (Figure 1): fluid-attenuated inversion recovery highlighted diffuse, confluent, and symmetrical hyperintensities in periventricular and deep localizations, sparing U-fibers and basal ganglia. In posterior fossa, hyperintensities involved middle cerebellar peduncles and cerebellar white matter. These abnormalities were mildly hypointense on T1-weighted images. No lactate peak on MRI spectroscopy analysis was found. Spinal-cord MRI and cerebrospinal fluid (CSF) analysis were considered normal. As a drug-related disorder was suspected, the infliximab therapy was discontinued after a total duration of 10 months. All of the neurological symptoms then progressively disappeared over 1 year.
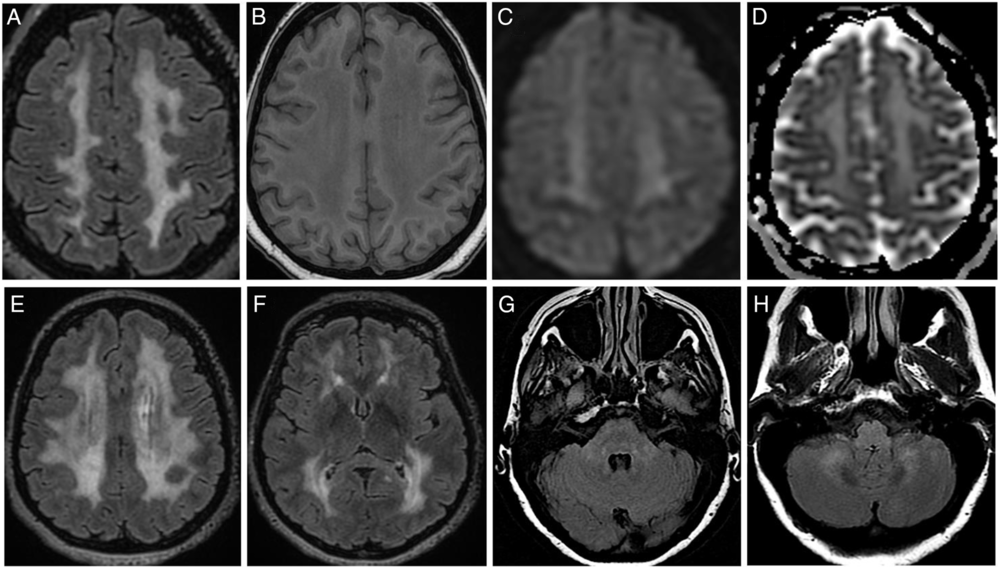
Figure 1: Brain MRI features. (A–D) Images of centrum semiovale area. Widespread hyperintensity on FLAIR sequence (A, E, F) with the corresponding T1w (B) hypointensity involving periventricular and deep white matter areas. Basal ganglia and subcortical U-fibers appear grossly spared (F). Diffusion-weighted imaging (C) and apparent diffusion coefficient (D) show a hyperintensity corresponding to T2-shine-through phenomenon. Subtle hyperintensity on FLAIR sequence within the pons (G) and cerebellum (H). FLAIR=fluid-attenuated inversion recovery; T1w=T1-weighted.
At the age of 49, she required an ileocecal resection with an ileostomy because of small bowel obstruction secondary to an ileal stricture. Histopathology revealed features suggestive of CD: mucosal ulcers sometimes complicated with fissures and fistulas, transmural inflammation, but no epithelioid granuloma.
She was referred to our Department of Neurology at the age of 50 for the assessment of the WMH, which remained stable 3 years after the first performed MRI, contrasting with the clinical improvement. Clinical examination found only areflexia and decreased vibratory sensations in lower limbs. Cognitive functions were preserved. A nerve conduction study (NCS) revealed a diffuse and homogeneous slowing of motor conduction velocities without conduction block that was predominant in the lower limbs (30–35 m/s), prolonged distal latency of the right peroneal (5.6 ms) and both tibial nerves (6.1 and 6.9 ms), and a slowing of F-waves (30 m/s in lower limbs and 35 m/s in upper limbs). Motor and sensory amplitudes were reduced in the lower limbs. These results were consistent with demyelinating peripheral neuropathy and secondary axonal loss.
Combined central and peripheral demyelination suggested a genetic disease. Owing to the gastrointestinal-associated symptoms with a poor response to therapeutics used for CD and to the familial history, we suspected an MNGIE. The muscle biopsy revealed ragged-red fibers and cytochrome oxidase negative fibers. Lymphocytic thymidine phosphorylase activity was undetectable (<0.05 [0.48–0.96 μmol/h/mg protein]), and plasma thymidine and 2′ deoxyuridine were high, respectively, 1.3 μM (<0.5) and 3.2 μM (<0.5), which confirmed an MNGIE. Sequencing of TYMP gene found a new homozygous variant c.211A>G (p.Ile71Val) in exon 2, which had never been reported in Exome Sequencing Project and Exome Aggregation Consortium databases. This variant showed equivocal damage on protein function according to prediction software (PolyPhen-2), and its position was mildly conserved. A postmortem genetic analysis of TYMP gene was performed in her sister and found the same homozygous variant, therefore highly suggestive of being pathogen.
Findings of brain MRI WMH in a patient with CD should lead to investigate several specific causes (Table 1). A demyelinating disease should be first considered, because of its epidemiological association with CD. Multiple sclerosis (MS) and demyelinating peripheral neuropathies were reported to be more frequent in inflammatory bowel disease patients than in the general population, independently of any anti-TNF-α therapy.Reference Kosmidou, Katsanos and Katsanos1 Besides, TNF-α blockers such as infliximab, which are commonly used to treat CD, are associated with demyelinating diseases (MS, peripheral neuropathy) as an adverse effect.Reference Bosch, Saiz and Ramos-Casals2 In our case, the lack of oligoclonal bands in the CSF, the contrast between diffuse WMH and minor neurological disability, and the peculiar pattern of WMH made the MS (drug-related or not) diagnosis unlikely. Regarding the peripheral neuropathy, the very homogeneous slowed motor nerve velocities and the absence of elevated CSF protein content were evidence against the diagnosis of an acquired demyelinating neuropathy. Besides, NCS abnormalities were found more than 2 years after the infliximab discontinuation, which made a drug-related neuropathy unlikely. However, the disappearance of neurological symptoms after its discontinuation remains misunderstood but might be due to a transient superimposed infliximab-induced peripheral neuropathy.
Table 1: Radiological and clinical features of leukoencephalopathies associated with Crohn’s or Crohn’s-like disease

CNS = central nervous system; IBD = inflammatory bowel diseases.
Cases of progressive multifocal leukoencephalopathy (PML) in patients treated with TNF-α blockers were described.Reference Bosch, Saiz and Ramos-Casals2 Spared U-fibers, the absence of disease progression on MRI, and the clinical improvement were not in favor of PML diagnosis, which was not tested in the CSF.
Vitamin deficiencies are frequent in CD because of impaired nutrient absorption and can lead to demyelination of both central and peripheral nervous systems, in particular, vitamin B12 and folic acid.Reference Pan, Liu and Guo3 The tests we made were normal (B12: 314 pmol/L [normal values 140–490]; acid folic: 42 nmol/L [9–61]; homocysteine: 10.9 μmol/L [4.5–13.5]). Methylmalonic acid was not tested.
MNGIE is an autosomal recessive disorder caused by mutations in the thymidine phosphorylase gene (TYMP, NM_001113755.1). Symptoms usually appear before the age of 20 with gastrointestinal dysmotility that may lead to a chronic intestinal pseudo-obstruction. Progressive external ophthalmoplegia, peripheral neuropathy, and leukoencephalopathy are also frequent manifestations. Of note, the leukoencephalopathy often remains asymptomatic despite its diffuse pattern and seems to be a constant feature of MNGIE.Reference Garone, Tadesse and Hirano4 MNGIE is not a cause of CD, but interestingly a CD-like presentation as in our patient, including histology features, is increasingly described in MNGIE patients.Reference Patel, Coulter, Rimmer, Parkes, Chinnery and Swift5 Intestinal inflammation may be secondary to the dysmotility and/or linked to the mitochondrial dysfunction of intestinal cells. Currently, MNGIE has no approved treatment, but several therapeutic strategies were tested and are under further investigations.
In summary, unexplained brain WMH in patients with presumed CD should trigger suspicion of MNGIE and lead to test thymidine phosphorylase activity (Supplementary figure).
Acknowledgments
The authors thank the patient for agreeing to the publication.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
LC and YN collected the data, drafted, and revised the manuscript. VB, NS, XT, RB, and PG revised the manuscript. NS also drafted the figure.
Standard Protocol Approvals, Registrations, and Patient Consent
This study was conducted according to the French legislation and authorized by CNIL committee (No. 2211991). Written informed consent was obtained from the patient.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.41.






