Introduction
Patients with schizophrenia are inclined to discontinue antipsychotics once remission is achieved for a variety of reasons [Reference Perkins, Gu, Weiden, McEvoy, Hamer and Lieberman1], but current evidence does not favor this discontinuance due to concerns about the higher risk of relapse [Reference Correll, Rubio and Kane2] and poorer prognosis once relapse has occurred [Reference Hui, Honer, Lee, Chang, Chan and Chen3, Reference Takeuchi, Siu, Remington, Fervaha, Zipursky and Foussias4]. While valid predictors to identify individuals who can discontinue medication successfully are scarce [Reference Alvarez-Jimenez, Priede, Hetrick, Bendall, Killackey and Parker5, Reference Bowtell, Ratheesh, McGorry, Killackey and O’Donoghue6], clinicians’ attitudes towards discontinuing antipsychotics in patients with remitted psychosis are a mix of anticipation and apprehension [Reference Thompson, Singh and Birchwood7–Reference Hui, Wong, Leung, Lee, Chan and Chang9]. The debates continue between maintenance versus discontinuation of antipsychotics for stable patients because no consensus has been reached while weighing the detrimental results of relapse following medication discontinuation [Reference Correll, Rubio and Kane2, Reference Hui, Honer, Lee, Chang, Chan and Chen10, Reference Tiihonen, Tanskanen and Taipale11], and the potential hazards of long-term antipsychotic treatment on patient’s physical health, cognitive functioning, and even brain structure [Reference Fusar-Poli, Smieskova, Kempton, Ho, Andreasen and Borgwardt12–Reference Vita, De Peri, Deste, Barlati and Sacchetti15].
Dose-reduction was expected to be a compromise solution for this debate [Reference Liu and Takeuchi16], and a few studies have reported findings supporting this approach [Reference Ozawa, Bies, Pillai, Suzuki, Mimura and Uchida17–Reference Huhn, Leucht, Rothe, Dold, Heres and Bornschein19]. The dose-reduction trials of Uchida et al. found that the therapeutic window of D2 receptor blockade could be lower than 65% for stable patients [Reference Tsuboi, Suzuki, Bies, Remington, Pollock and Mimura20–Reference Uchida, Suzuki, Graff-Guerrero, Mulsant, Pollock and Arenovich22]; accordingly, the dose during maintenance should be lower than the commonly recommended levels. Even though a meta-analysis suggested that the risk of relapse in patients undergoing dose reduction is twofold of their maintenance counterparts, neurocognitive functioning in the former is significantly improved after dose reduction [Reference Tani, Takasu, Uchida, Suzuki, Mimura and Takeuchi23]. A recent systematic review reported similar findings and called for more attention to this approach concerning its potential to improve patients’ quality of life and global functioning [Reference Rodolico, Siafis, Bighelli, Samara, Hansen and Salomone24].
While guidelines advise that patients may try to discontinue antipsychotics once they have been remitted from a first psychotic episode [Reference Shimomura, Kikuchi, Suzuki, Uchida, Mimura and Takeuchi25], no guideline has explicitly addressed how to achieve this goal safely. A few longitudinal studies have presented favorable long-term outcomes of patients who remained drug-free, clinically stable, and with good functioning [Reference Wils, Gotfredsen, Hjorthoj, Austin, Albert and Secher26–Reference Harrow, Jobe and Faull28], yet they have provided little information regarding how to reach such a favorable status. On the other hand, several medication discontinuation trials that endorsed maintenance therapy may have overestimated the risk of relapse, either discontinuing antipsychotics too fast, which rendered patients at risk of dopamine supersensitivity psychosis [Reference Chouinard, Samaha, Chouinard, Peretti, Kanahara and Takase29], or monitoring symptoms too closely after medication discontinuation, resulting in misrecognizing symptoms associated with antipsychotic withdrawal as a form of relapse [Reference Horowitz, Murray and Taylor30]. Thus, a closer look at dose tapering is needed to examine the underpinnings.
The pioneering work of Wunderink et al. regarding guided antipsychotic discontinuation revealed that 46.2% of subjects did not completely stop antipsychotics within 18 months; among those who had once reached discontinuation, only 21.5% remained in a remitted state, 24.6% restarted antipsychotics due to relapse, and 7.7% restarted medication once mild recurrent symptoms re-emerged [Reference Wunderink, Nienhuis, Sytema, Slooff, Knegtering and Wiersma31]. In a small pilot study of patients with remitted chronic psychosis, Huhn et al. illustrated detailed 6-month trajectories of individuals undergoing dose tapering [Reference Huhn, Leucht, Rothe, Dold, Heres and Bornschein19]. Gaebel et al. used graphic presentations, including relevant clinical information, in representative case vignettes to depict long-term treatment with antipsychotics [Reference Gaebel, Stricker and Riesbeck32]. Their works provide a framework by which to exemplify the dynamic processes during dose-tapering, which is pivotal in developing a guided dose-reduction algorithm to reach a desirable and balanced risk-to-benefit ratio.
Several clinical trials were initiated to test whether or not it is feasible to conduct medication reduction/discontinuation for better functioning outcomes [Reference Moncrieff, Lewis, Freemantle, Johnson, Barnes and Morant33–Reference Begemann, Thompson, Veling, Gangadin, Geraets and t Hag36]. Likewise, our team conducted a prospective comparative cohort study from August 2017 to September 2022 in Taiwan to test the protocol of guided antipsychotic reduction to reach minimum effective dose (GARMED) [Reference Liu, Hsieh, Chien, Liu, Lin and Hwang37]. Different from previous trials which aimed to discontinue antipsychotics completely while accepting an increased risk of relapse as the tradeoff, the goal of GARMED was to challenge whether stable patients could successfully reduce doses without increased risk of relapse. As shown by our previous observations in a naturalistic cohort [Reference Liu, Liu, Chien, Lin, Hsieh and Hwang38], we expected the present trial to reach a dose much lower than the minimum effective dose (MED) estimated by doses that exert a distinguishable improvement in symptoms shown in randomized, double-blind, fixed-dose trials comparing antipsychotics to placebo [Reference Leucht, Samara, Heres and Davis39, Reference Woods40].
Currently, our GARMED trial revealed that the relapse rate of the guided dose reduction (GDR) group was not higher than their maintenance counterparts [Reference Liu, Hsieh, Chien, Liu, Lin and Hwang41]. Among the 51 GDR patients in this study, 74.5% stayed well and relapse-free under a dose lower than their baseline level, while not all of them could reduce doses successively as planned during a 2-year observation-GDR trial. On the other hand, 25.5% of GDR patients were not able to reduce any dose, either having a relapse or having to resume their baseline dose to prevent relapse during dose tapering. In this report, we focused on examining GDR patients with specific interests to (1) depict and subgroup individual trajectories during the course of dose reduction, (2) compare baseline demographic and clinical characteristics between patients with different trajectories, and (3) explore factors associated with dose tapering patterns.
Methods
Design, setting, and participants
This open-label randomized prospective comparative cohort trial was based on a previously described pragmatic design (Figure 1) [Reference Liu, Hsieh, Chien, Liu, Lin and Hwang37]. In brief, patients with remitted psychosis (a history of schizophrenia-related psychotic disorders based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5] diagnosis) under treatment with a stable antipsychotic dose over 3 months were eligible (see eligible criteria in Supplementary Table S1). After being briefed with a psychoeducation session regarding the rationale for this GDR trial and possible risks and benefits during tapering, two-thirds of eligible patients willing to try guide dose reduction were randomized to GDR group. All included patients provided signed informed consent to participate before starting the trial. Patients younger than age 20 years provided signed consent from their parents or guardians in accordance with Taiwan law. Ethical approval was obtained from the Research Ethics Committee of the study hospital (REC: 201703002RIND), and this study was registered at ClinicalTrials.gov (identifier: NCT03248180).

Figure 1. Diagram of trial flow chart.
Dose-reduction process
The rationale and details of the dose-reduction algorithm were also detailed in previous papers [Reference Liu and Takeuchi16, Reference Liu, Hsieh, Chien, Liu, Lin and Hwang37]. In brief, the extent of dose reduction is small (reducing no more than one-quarter of the current dose at a time), and the tempo of tapering is slow (the next dose reduction attempt would not be initiated until 24 weeks of stabilization). In addition, patients needed to be monitored every 4 weeks for three consecutive sessions once undergoing dose reduction, and they would stay at the same dose for another 12 weeks if maintained stabilized, defined as no aggravation of symptoms lasting for more than 1 week during this time span.
Importantly, patients received instructions regarding suspected symptoms of relapse during tapering, the possibility of a need to resume a rescue dose, and how to call for help when not sure about their subjective changes. In such cases, extra return visits were arranged and patients were supervised to resume doses at the previous level if experiencing suspected signs of relapse. If any one of the 3 positive symptoms and 2 general symptoms in the inclusion criteria could not be stabilized within 1 week (i.e., scores >3) under their baseline antipsychotic dose, the patient would be treated with a higher dose and designated as having a relapse. If maintained stable under a reduced dose for 24 weeks, patients were empowered with a shared decision-making process regarding whether they wished to try another dose reduction or not. Patients could decide to stay at the current dose for a more extended time if feeling not ready for further dose tapering at the designated timepoint.
The subsequent dose reduction was a reiteration of the previous step, cutting off one-quarter of the current dose, yielding 9/16 (3/4 × 3/4) of the baseline dose (in a hyperbolic manner), rather than cutting off another 1/4 of the baseline dose (in a linear manner), as the latter would result in reducing 1/2 (1/4 + 1/4) of the baseline dose after two dose-reductions and end at zero by employing four dose reducing steps, while the first formula, (3/4)n, is able to be re-iterated indefinitely—a strategy feasible for minimizing the negative impact of dopamine-supersensitivity [Reference Horowitz, Jauhar, Natesan, Murray and Taylor42]. Also, the actual daily doses taken during tapering would not always fit precisely with the numbers calculated by this formula. The latter just served as the minimum dose for each timepoint since it was impractical to cut off a quarter or even smaller piece of a tablet for daily dosing [Reference Liu, Hsieh, Chien, Liu, Lin and Hwang37].
Depicting and subgrouping trajectories of dose tapering
To illustrate individual trajectories of dose tapering in a consistent manner, the doses at each timepoint of individual patient were first transformed as the proportions of their baseline doses; that is, the baseline level was designated as “1”, uniformly across all patients, regardless of which antipsychotics and doses were received at the beginning of this trial. Second, the current dose/baseline dose was calculated to make up plots comprising all fractions of doses at each timepoint of the individual patients, in order to portray a constellation of trajectories for all patients using a standardized visual presentation. Then, the patterns of patients could be visually identified straightforwardly based on their trajectories during dose tapering, as follows:
1) Sequential reducer (SR): Applied to those who tapered doses successfully at each designated timepoint for 4 steps in 2 years; 2) Modest reducer (MR): Applied to those who took a cautious pace in tapering, only reducing 1 to 3 steps, with no re-escalation to previous steps, during 2 years; 3) Alert reducer (AR): Applied to those who reduced at least 2 steps yet re-escalated the dose back to the level between their baseline dose and the lowest dose reached during the dose-tapering course; 4) Baseline returner (BR): Applied to those who tapered at least 1 step but returned to the baseline dose due to concern about risk of relapse and were able to stay in remission throughout the course; 5) Failed reducer (FR): Applied to those who had a relapse during dose tapering and were in need of a dose higher than their baseline level; 6) Early exit (EE): Applied to those who withdrew from the trial after tapering at least 1 step without relapse before the end of 2-year follow-up.
Examples of subjects with each pattern are illustrated in Figure 2. Constellations of all individual patient’s trajectories are presented in Supplementary Figure S1. The modest reducers, alert reducers, and baseline returners were further collapsed as a subgroup of “conservative reducers (CR)” to be compared with the other 3 subgroups.

Figure 2. Patterns of dose tapering trajectories. Sequential reducers (SR), those who were able to taper doses at each designated timepoint successfully for four steps in 2 years; Modest reducer (MR), those who took a cautious pace in tapering, thus they only reduced 1 to 3 steps, with no re-escalation to previous step, during 2 years; Alert reducer (AR), those who have had reduced at least two steps yet re-escalated dose back to the level between their baseline dose and the lowest dose they have reached during the course of dose tapering; Baseline returner (BR), those who have tapered at least 1 step but returned to baseline dose for concern about risks of relapse and were able to stay in remission throughout the course; Failed reducer (FR), those who had a relapse during dose tapering and were in need of a dose higher than their baseline level; Early exit (EE), those who left the trial after tapering at least 1 step without relapse before the end of 2-year follow-up. AR, BR, and MR were collapsed as conservative reducers (CR) in further analyses.
Clinical assessments
Clinical severities were rated using the Mandarin version Positive and Negative Syndrome Scale (PANSS) [Reference Cheng, Ho, Chang, Lane and Hwu43], Clinical Global Impression of Severity (CGI-S), and Personal and Social Performance scale (PSP) [Reference Wu, Lin, Tseng, Liu, Chen and Huang44], which were carried out by each patient’s attending psychiatrist. Patients’ employment status was collected by face-to-face interview with our research assistant. Patients’ subjective experiences were reported by filling out a medication satisfaction questionnaire (MSQ) based on a 7-point Likert scale [Reference Vernon, Revicki, Awad, Dirani, Panish and Canuso45], and using the EuroQoL-5D visual analogue scale (EQ-5D-VAS) to evaluate quality of life [Reference Chang, Lin, Chang, Chen, Huang and Liu46]. In addition, each patient’s medical records were reviewed to collect demographics, clinical diagnosis, age of onset, illness duration, and any history of psychiatric hospitalization and psychotic relapse during their illness.
Statistics
To examine between-group differences, baseline clinical characteristics were compared between groups using χ2 tests for categorical variables and analyses of variance (ANOVAs) for continuous variables. Differences in scores between baseline and 2-year follow-up were compared using the paired t-test. To explore whether the patient with certain factors would more likely become a sequential reducer, conservative reducer, or failed reducer, univariate logistic regression followed by multivariate logistic regression analysis with backward stepwise selection of baseline clinical variables, and eliminating variables with a probability >0.2, were employed, respectively. Hypothesis testing and confidence intervals were two-sided at the 5% significance level (STATA 13; Stata Corp., College Station, Texas, USA).
Results
Baseline characteristics
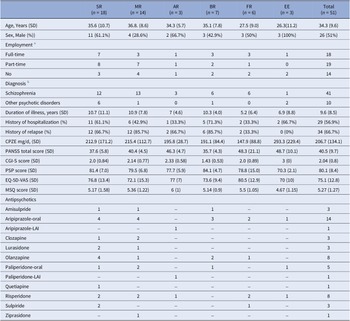
The diagram of this trial is illustrated in Figure 1. Among the 51 patients assigned to guided dose-reduction group, there were 18 sequential reducers, 14 modest reducers, 3 alert reducers, 7 baseline returners, 6 failed reducers, and 3 early exits. The details of baseline demographic and clinical characteristics among 6 patterns of trajectories are presented in Table 1. Comparisons of demographic variables and changes between baseline and end-of-follow-up clinical variables are displayed in Table 2.
Table 1. Baseline demographic and clinical characteristics of participants among 6 trajectories

Abbreviations: Naming subgroups: AR: alert reducer; BR: baseline returner; EE: early exit from follow-up; FR: failed reducer; MR: modest reducer; SR: sequential reducer; Baseline and clinical variables: CGI-S: clinical global impression-severity; CPZE: chlorpromazine equivalent dose; EQ-5D-VAS: EuroQoL-5D visual analogue scale; LAI: long-acting injectable; MSQ: medication satisfaction questionnaire; PANSS: positive and negative syndrome scale; PSP: personal and social performance.
a Full-time employment defined as having a job >15 h per week for more than 3 months during the past 6 months; Part-time employment defined as serving some duties or having a job but not meeting the requirement for full-time employment.
b Schizophrenia included schizophrenia and schizophreniform disorder; Other psychotic disorder included other schizophrenia-spectrum and other psychotic disorder based on DSM-5 criteria.
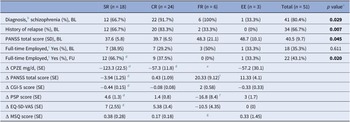
Table 2. Comparisons of demographic/clinical characteristics and changes between baseline and 2-year follow-up across 4 subgroups

Abbreviations: Subgroup naming: SR: sequential reducer; CR: conservative reducer (comprising MR modest reducer, AR alert reducer, and BR baseline returner); FR: failed reducer; EE: early exit from follow-up; Baseline and clinical variables: BL: baseline; CGI-S: clinical global impression-severity; CPZE chlorpromazine equivalent dose; ∆: within-subgroup differences between the end of follow-up and baseline examined by paired t-test; EQ-5D-VAS: EuroQoL-5D visual analog scale; FU: end of follow-up status; PANSS: positive and negative syndrome scale; PSP: personal and social performance; SD: standard deviation; SE: standard error.
a p value denotes the statistics among 4 subgroups.
b Schizophrenia includes schizophrenia and schizophreniform disorder, versus other schizophrenia-spectrum and other psychotic disorders.
c Full-time employment is defined as having a job >15 hours per week for more than 3 months during the past 6 months.
d Within a subgroup, the end-of-follow-up scores were statistically significantly different from their baseline scores compared to paired t-test.
e Two patients actually stopped medications on their own accord, the other four patients had relapsed and even resumed a dose higher than the baseline level.
f Excluding a case who started from exceptionally high baseline PANSS scores and then steadily decreased severity during the course until relapse, the remaining 5 failed reducers showed significant worsening in PANSS and PSP comparing their scores at baseline and at the time of relapse by paired t-test.
g Patients having a relapse did not report MSQ by that time.
Statistics with p values < 0.05 were displayed in bold.
The low baseline severity and relatively high personal social functioning suggest patients’ remitted states at the beginning of dose tapering. Durations of illness ranged widely from 0.8 to 35 years (mean 9.6 ± 8.5 years), and the majority had a history of psychiatric hospitalization (56.9%) and a history of relapse (66.7%). Baseline treatment comprised 10 oral and 2 long-acting injectable antipsychotics with dose ranges skewed to the lower end of each agent, with an average CPZE dose of around 200 mg/d (Table 1 and details in Supplementary Figure S1). Distribution of different antipsychotics was scattered across all subgroups, while oral-form aripiprazole (n = 14) was the most prevalent, followed by a tie between olanzapine and risperidone (both n = 8). Statistically significant differences were found between the four subgroups in diagnosis of schizophrenia (versus other schizophrenia-spectrum psychotic disorders), history of relapse, and baseline symptom severity (Table 2).
Changes of clinical characteristics after 2-year follow-up
By the end of 2-year follow-up, SR patients on average reduced 58.5% of baseline doses and stayed well under an average post-reduction dose CPZE 89.6 ± 79.7 mg/d, while statistically significant improvement was seen in symptom severity, clinical global impression, personal social functioning, and subjective quality of life. More SR patients were also able to have a full-time job. No such significant changes were seen in CR patients and FR patients (Table 2).
Factors associated with different dose-tapering patterns
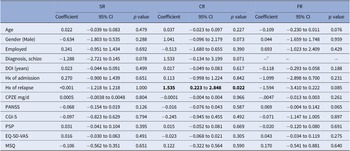
Univariate logistic regression analyses of factors associated with each trajectory revealed that a history of relapse was significantly associated with the category of conservative reducer (odds ratio (OR): 4.64, 95% confidence interval (CI): 1.25–17.25, p = 0.022) (Table 3). Besides, diagnosis of schizophrenia was marginally related to increased likelihood of being a conservative reducer (OR: 4.63, 95% CI: 0.87–24.52, p = 0.071) yet decreased likelihood of being a sequential reducer (OR: 0.28, 95% CI: 0.07–1.16, p = 0.078), while all 6 FR patients were with the diagnosis of schizophrenia. Results were examined further using multivariate logistic regression analysis with backward stepwise selection to see if any model provided additional information (Table 4).
Table 3. Univariate logistic regression analyses for factors related to designated trajectories

Abbreviations: Subgroup naming: SR: sequential reducer; CR: conservative reducer, comprised by MR, AR and BR; FR: failed reducer; Baseline and clinical variables: BL: baseline; CGI-S: clinical global impression-severity; CPZE chlorpromazine equivalent dose; EQ-5D-VAS: EuroQoL-5D visual analogue scale; FU: end of follow-up status; PANSS: positive and negative syndrome scale; PSP: personal and social performance; SD: standard deviation; SE: standard error.
a All 6 FR patients were with the diagnosis of schizophrenia.
Statistics with p values < 0.05 were displayed in bold.
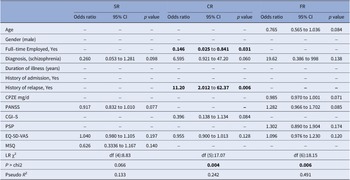
Table 4. Multivariate logistic regression analyses for factors related to designated trajectories

Abbreviations: Subgroup naming: SR: sequential reducer; CR: conservative reducer, comprising MR, AR and BR; FR: failed reducer; Baseline and clinical variables: BL: baseline; CGI-S: clinical global impression-severity; CPZE chlorpromazine equivalent dose; EQ-5D-VAS: EuroQoL-5D visual analogue scale; FU: end of follow-up status; PANSS: positive and negative syndrome scale; PSP: personal and social performance; SD: standard deviation; SE: standard error.
Statistics with p values < 0.05 were displayed in bold.
Multivariate analysis showed that the relationship between history of relapse and being a conservative reducer was more pronounced (OR: 11.20, 95% CI: 2.012–62.37, p = 0.006). Moreover, patients with a full-time job at baseline were significantly less likely to become conservative reducers (OR: 0.146, 95% CI: 0.03–0.84, p = 0.031). In the multivariate model, the factors of having a history of relapse, and not having a full-time job, together with a diagnosis of schizophrenia, lower clinical global severity and lower quality of life, was significantly predictive of becoming conservative reducers (likelihood ratio (LR) χ2, degree of freedom (df) (5): 17.07; p = 0.004; Pseudo R 2: 0.242).
Only 6 failed reducers were identified in this cohort, all with a diagnosis of schizophrenia yet no other variable was found to be significantly associated with relapse during dose tapering. Multivariate logistic regression analyses showed that the model composed of younger age, lower baseline antipsychotic dose, higher baseline PANSS score, higher personal social functioning, better quality of life, and having a full-time job was significantly associated with relapse during drug tapering (LR χ2, df (6):18.15; p = 0.006; Pseudo R 2 = 0.4913).
Univariate logistic regression also failed to find any demographic or clinical variable with a significant impact on the chance of being a sequential reducer. The model with the lowest probability as checked by LR Chi2, composed of a lower baseline PANSS score, lower medication satisfaction, with a diagnosis of other schizophrenia-spectrum psychotic disorder, and higher quality of life, was still not significantly predictive for indicating a sequential reducer (LR χ2, df (4):8.83; p = 0.065; Pseudo R 2 = 0.133).
Discussion
This study is one of the few to depict dose-tapering trajectories for stable patients receiving antipsychotic maintenance treatment [Reference Huhn, Leucht, Rothe, Dold, Heres and Bornschein19, Reference Wunderink, Nienhuis, Sytema, Slooff, Knegtering and Wiersma31, Reference Gaebel, Stricker and Riesbeck32]. Our results suggest that it is a long and winding road to attempt discontinuation of antipsychotics. Based on a study design that empowered patients to participate in shared decision-making when eligible to take further dose reduction, or to re-escalate dose to the previous level once having suspected signs of relapse, results of this study can serve as valuable references to studies and practice in a real-world setting [Reference Relton, Torgerson, O’Cathain and Nicholl47].
Even though the algorithm in this study guided clinicians to taper antipsychotics very slowly and in a hyperbolic manner as suggested previously [Reference Horowitz, Jauhar, Natesan, Murray and Taylor42], only 1 out of 3 patients (18/51) was able to reduce a quarter of the current dose consecutively every 6 months, repeating it four times in 2 years, without experiencing a relapse. The average post-reduction dose of these SR patients, CPZE 90 mg/d, is much lower than the dose, 200 mg/d, identified to be an important factor associated with successful dose reduction by a meta-analysis [Reference Tani, Takasu, Uchida, Suzuki, Mimura and Takeuchi23]. SR patients reached a significantly improved condition in many aspects of life, instilling hope for patients remitted from psychosis to pursue such desirable outcomes. However, no clinical variable was identified that helps to predict who would have a better chance to reach this ideal trajectory. Thus, individualized patient care appears to be the key to successful dose-tapering. Also, a sound support system is an important protective factor that was not formally recorded as a covariate in this study, and it deserves better attention in future studies.
The relatively low relapse rate of this cohort, not higher than their maintenance counterparts [Reference Liu, Hsieh, Chien, Liu, Lin and Hwang41], may be a result of many patients (24/51) bearing a cautious and conservative stance towards dose tapering. They either opted to stay at a slower pace (MR), or re-escalate to a higher dose whenever unsure if warning signs were re-emerging (AR or BR), as to prevent a full-blown relapse. Such a cautious attitude correlates highly with the fact that 5 out of 6 CR patients have a history of relapse during previous treatment. Patients in this subgroup on average reduced 28% of baseline dose in 2 years (40, 29, and 0% for MR, AR, and BR, respectively), while no substantial improvements in symptoms and quality of life were attained (Table 2). This result may raise questions about whether it is worth investing good efforts for tapering doses with such a modest gain. Nevertheless, as long as they could maintain stable remission, in the future there is still a chance to reduce the dose by tapering a smaller dose at a time and allowing longer observation if suspected re-emerging warning signs will wane without re-escalating the dose.
Although only a small number of patients experienced relapse during dose tapering, several factors for precaution were identified. In addition to the diagnosis of schizophrenia, a well-known risk factor of relapse [Reference Bowtell, Ratheesh, McGorry, Killackey and O’Donoghue6], the higher baseline PANSS score suggests symptom stability is still an important prerequisite when considering dose tapering. Interestingly, higher personal social functioning, better quality of life, and having a full-time job were linked to increased risks of relapse, suggesting that these patients might overlook the risk of relapse because they had been doing quite well at the entry of the study. Indeed, two patients with good functioning and quality of life confessed that they had stopped taking antipsychotics completely on their own accord, leading to a relapse soon after their discontinuation attempts. Additionally, a lower baseline antipsychotic dose is likely to contribute to a higher risk of relapse, which is in accord with the hyperbolic dose–response curves of antipsychotic dose and relapse prevention which illustrates a disproportionately increased risk of relapse at the lower dose end [Reference Leucht, Bauer, Siafis, Hamza, Wu and Schneider-Thoma48], as well as the prediction of Horowitz et al. that it will be easier to taper a larger fraction of medication at higher dose levels, while it will be safer to only cut off a much smaller fraction of dose at the lower dose level [Reference Horowitz, Jauhar, Natesan, Murray and Taylor42]. This phenomenon deserves better attention as it will be crucial if wishing to discontinue antipsychotics completely with a favorable ending.
As the sample size of each subgroup is small and not feasible for sophisticated statistical analyses, this study was more exploratory than confirmatory, so several limitations must be addressed. First, most participants were cooperative with this delicate tapering procedure, implying that they were in a better position to anticipate a good individual prognosis, thus the results may not be generalizable to patients who were not able to follow the guided processes reliably. Second, most patients had more than one psychotic episode, a condition in which most guidelines do not usually recommend discontinuing antipsychotic medications [Reference Shimomura, Kikuchi, Suzuki, Uchida, Mimura and Takeuchi25]. Thus, as indicated previously [Reference Yen, Liu, Lin, Chien, Hsieh and Liu8], not only patients but also their doctors were more likely to adopt a cautious attitude during dose tapering, which may inflate the proportion of patients categorized in a conservative group. Third, to minimize the risk of relapse, the algorithm defined the threshold of relapse as having symptoms aggravated for more than 1 week. However, this may be too sensitive to capture those who were able to accommodate antipsychotic-associated withdrawal symptoms and regain stabilization without an increased dose after 1 week [Reference Horowitz, Murray and Taylor49], again resulting in an exaggerated proportion of patients staying with a conservative trajectory yet preventing them to reduce more doses which might be achievable via a more flexible tapering approach [Reference Horowitz, Moncrieff, de Haan, Bogers, Gangadin and Kikkert50, Reference Horowitz and Taylor51]. Lastly, the relatively small sample sizes in each pattern did not allow sophisticated statistical analyses; three trajectories even had to be collapsed into one subgroup (CR), while subtle yet important differences may exist among MR, AR, and BR patients.
Echoing the advocacy of Marder and Zito: “We encourage a sense of curiosity about the possibility of dose reduction and discontinuation in appropriate patients.” [Reference Marder and Zito52], as well as the advocacy of Murray and Di Forti: “Guidelines need to be developed on when and how slowly to reduce antipsychotics, and in whom it is appropriate to eventually stop them.” [Reference Murray and Di Forti53], this report employs a visual presentation to recognize and define the patterns of dose-tapering trajectories along a zigzag path. The proposed naming provides a framework to depict individual patient’s course. To develop guidelines for delivering better clinical practice to this patient population, researchers can adopt such an intuitive descriptor system, examine the progress of patient’s dose reduction with a clear view portrayed by reasonable anchor points, and compare findings based on a common ground. Also, clinicians can discuss with their remitted patients by referring to the visual illustration of dose-tapering processes as well as highlighting the pros, cons, and warnings during shared decision-making. Currently, three-fourths of this cohort agreed to receive follow-up by our research team and we will monitor their trajectories and outcomes via this lens in the longer term.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1192/j.eurpsy.2023.2440.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
We appreciate Dr. Ko Yen for his assistance with ascertaining medical records and Dr. Tzu-Yu Liu for the artwork of graphs. This work has been presented at an online international meeting “Antipsychotic medication discontinuation and reduction trials” and we appreciate feedback from the members of the TAPER (Tapering AntiPsychotics and Evaluating Recovery) Research Consortium.
Author contribution
C-C.L. contributed to the conception, design, and initial drafting of the manuscript. C-C.L., M.M.H., Y-L.C., C-M.L., Y-T.L., and T-J.H. contributed to data acquisition. C-C.L. conducted the statistical analyses. All authors contributed to the interpretation of results, the writing of the paper, and reading and approving the final manuscript.
Financial support
This study was supported by research grants MOST 106–2314-B-002-106, 107–2314-B-002-222-MY3, and 110–2314- B002–121-MY3 from the Ministry of Science and Technology (MOST), Taiwan. The MOST peer-reviewed this study protocol before granting the funding but played no role in the conduct of this project and had no involvement in the preparation, review, approval, or decision to submit this manuscript for publication.
Competing interest
The authors declare none.







Comments
No Comments have been published for this article.