Surgical site infections (SSIs) are the most common and expensive healthcare-associated infections, with attributable annual costs of US$3.2 billion in acute-care hospitals.Reference Merkow, Ju and Chung1,Reference Zimlichman, Henderson and Tamir2 Colorectal surgery has among the highest SSI rates, ranging from 15% to 30% in some studies.Reference Tanner, Khan, Aplin, Ball, Thomas and Bankart3,Reference Hubner, Diana, Zanetti, Eisenring, Demartines and Troillet4 The American College of Surgeons and Surgical Infection Society (ACS/SIS) have updated their clinical practice guidelines to include up to 20 specific interventions for SSI prevention in the pre-, intra-, and postoperative phase of surgical care.Reference Ban, Minei and Laronga5 Many hospitals have implemented some of these interventions within colorectal SSI prevention “bundles,” although the interventions that should be prioritized for bundle inclusion remains unclear. Our primary objective was to identify the colorectal bundle size and composition of guideline-recommended interventions most associated with SSI reduction. Our secondary aims were (1) to determine the impact of colorectal SSI prevention bundles on SSI incidence; (2) to describe the implementation strategies used; and (3) to assess adherence rates achieved with bundle implementation.
Methods
Search strategy
We systematically reviewed PubMed, Scopus, Cochrane, and the Cumulative Index to Nursing and Allied Health (CINAHL) for studies assessing SSI prevention bundles in colorectal surgery. We used the following keywords: colorectal surgery, digestive surgery, gastrointestinal surgery, surgical wound infection, surgical site infection, wound infection, enhanced recovery, fast track, eras, enhanced recovery after surgery, integrated control, multiple control, bundle, bundled, primary prevention, protocol, guideline adherence, policy compliance, quality improvement, clinical protocols, checklist, program evaluation, and program implementation. Two authors (C.A. and A.P.V.) independently screened the title with or without the abstracts of the references identified on the initial search and reviewed the full text of the articles eligible for inclusion. They also manually reviewed the references of retrieved articles to identify additional eligible studies. They resolved any discrepancies through discussion and consulting with a third author (N.S.) when appropriate. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines in conducting this systematic review.Reference Moher, Liberati, Tetzlaff, Altman and Group6
Inclusion and exclusion criteria
We included randomized controlled trials and observational studies up to December 2018, in any language, that assessed bundles composed of ≥3 interventions for the prevention of SSI among patients ≥18 years old undergoing colorectal surgeries. Studies that included colorectal surgery as part of complex surgeries involving other organs (eg, gynecology-oncology tumor debulking or hepatic surgery for metastatic resection) were excluded.
Data abstraction and assessment of study quality
Our main outcome was 30-day postoperative overall SSI rates before and after bundle implementation. We classified SSI outcomes as overall, deep, superficial, and organ-space, defined according to Centers for Disease Control and Prevention (CDC) criteria.Reference Horan, Gaynes, Martone, Jarvis and Emori7 We extracted the following information: authors and year of publication, study design, country, single versus multicenter study, interventions included in each bundle, number of patients in each study arm, and SSI rates for each study arm. We also collected data on adherence to individual and composite bundle interventions as well as strategies used to facilitate bundle implementation. We assessed risk of bias using the Downs and Black Checklist for Measuring Quality, which consists of 27 items evaluating reporting clarity, external validity, internal validity (bias, confounding), and power.Reference Downs and Black8 The maximum possible score, indicating the lowest risk of bias, is 31. Based on these scores, we assigned the following bias risk categories: high, for scores ≤19; medium, for scores 20–24; low, for scores 25–31; and indeterminate, for studies that did not provide enough raw data to calculate individual study power and compute a total score.
Statistical analysis
All publication data were extracted as raw data for analysis purposes. We excluded all studies that did not report raw numbers from the pooled analyses. We conducted a traditional meta-analysis procedure using the METAN statistical package in Stata statistical software.Reference Gilham9 We calculated pooled Mantel-Haenszel risk ratios (RRs), forest plots, and 95% confidence intervals (CIs) to assess the relationship between colorectal prevention bundle interventions and SSI reduction. A fixed-effects model was first used to pool SSI risk ratio estimates. If the fixed-effects model showed significant heterogeneity (ie, I2 > 50%), then a Der Simonian and Laird random-effects model was used to obtain adjusted standard errors of estimates and to lessen the level of variation. We estimated all study heterogeneity using Cochran’s Q statistics and I2. We examined the impact of bundle size and individual bundle components on SSI reduction using meta-regression and pairwise correlation coefficients. We graphically assessed publication bias using Egger funnel plots, Begg rank correlation, and Harbord tests. Further, the influence of individual studies was assessed using cumulative-effect models (METACUMReference Morgan, Pullon, Macdonald, McKinlay and Gray10 and METAINFReference Mays and Pope11). All P values <.05 were considered statistically significant. All analyses were conducted using Stata version 15 SE software (StataCorp, College Station, TX).
Results
Search results
Our initial search yielded 1,464 articles. After removing duplicates, we screened the titles and/or abstracts of 1,044 unique references. Of these, we reviewed the full texts of 57 articles, and we ultimately included 40 studies in our qualitative review, as summarized in the PRISMA flow diagram (Fig. 1).

Fig. 1. PRISMA flow diagram of literature search and study selection.
Study characteristics
Among these 40 selected studies, 3 (8%) were randomized-controlled trials,Reference Anthony, Murray and Sum-Ping12–Reference Ruiz-Tovar, Llavero, Morales and Gamallo14 29 (72%) were prospective studies,Reference Albert, Bataller and Masroor15–Reference Ohman, Wan and Guthrie43 and 8 (20%)Reference Bell44–Reference Keenan, Speicher, Thacker, Walter, Kuchibhatla and Mantyh51 were retrospective trials. Most studies (35, or 88%) were single-center studies, and 5 (12%) were multicenter trials. Study locations included the United States (n = 28), Spain (n = 3), Canada (n = 2), Japan (n = 2), England (n = 1), the Netherlands (n = 1), Italy (n = 1), Singapore (n = 1), and Australia (n = 1). Most studies (n = 33) reported overall SSI incidence (Table 1). Several studies did not report overall SSI incidence but did report superficial SSI incidence (n = 4),Reference Ruiz-Tovar, Llavero, Morales and Gamallo14,Reference Berenguer, Ochsner, Lord and Senkowski38–Reference Yamamoto, Morimoto and Kita40 and/or organ-space SSI incidence (n = 3)Reference Forbes, Stephen and Harper41,Reference Mammo, Peeples, Grodsky, Honaker and Wasvary42,Reference Keenan, Speicher, Thacker, Walter, Kuchibhatla and Mantyh51 (Table 1).
Table 1. Studies of Colorectal Infection Prevention Bundles and Their Effect on Surgical Site Infection (SSI) Rates
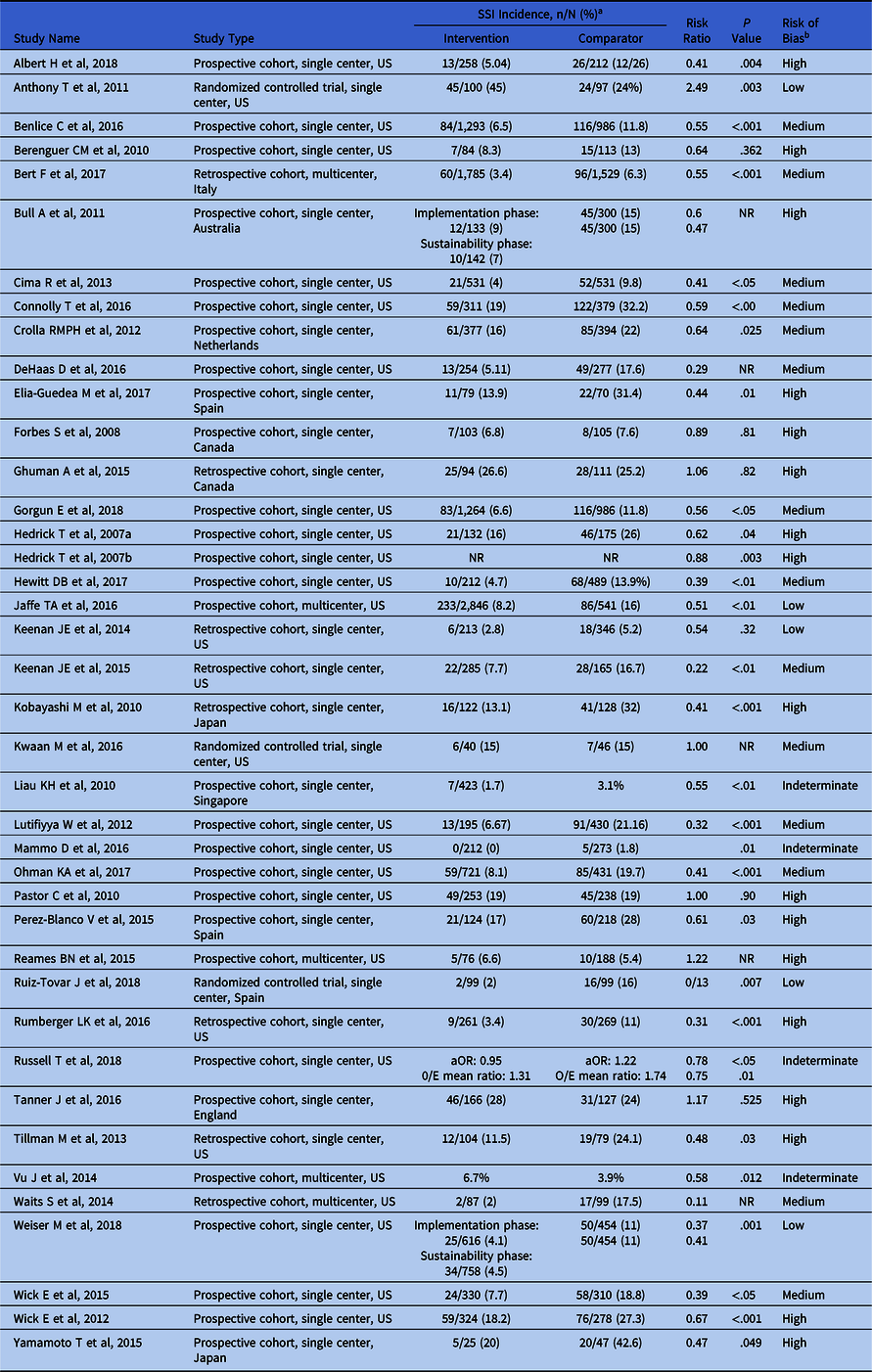
Note. aOR, adjusted odds ratio; O/E, observed/expected; NR, not reported.
a SSI outcomes are reported as superficial SSI for references 19, 41, 42, and 43; organ-space SSI for references 11, 44, and 45; and overall SSI for the remainder of the studies.
b Assigned based on Downs and Black score as follows: high (score ≤19); medium (score 20–24); low (score 25–31); indeterminate, if total score could not be calculated (studies did not report sufficient raw data).
Study quality and bias assessment
The risk of bias was low in 5 studies (12%), medium in 14 studies (35%), high in 17 studies (43%), and indeterminate in 4 studies (10%) (Table 1). The average Downs and Black individual study bias assessment score was 20.82 (range, 15–26). Of the 36 studies that provided enough raw data to allow power calculations, 18 (50%) were powered at <80%.
Composition of colorectal infection prevention bundles
There was significant heterogeneity in bundle composition across studies. The number of interventions recommended by the American College of Surgeons and Surgical Infection Society (ACS/SIS) clinical guidelines for SSI preventionReference Ban, Minei and Laronga5 varied between 2Reference Yamamoto, Morimoto and Kita40 and 13Reference Connolly, Foppa, Kazi, Denoya and Bergamaschi19,Reference Hewitt, Tannouri and Burkhart26 per bundle. The most common ACS/SIS bundle interventions were (1) intravenous antibiotic prophylaxis, present in 40 studies (100%), as standard of care, or with special emphasis on antibiotic choice and dosing as part of the intervention bundle; (2) normothermia maintenance in 32 studies (80%); (3) perioperative glucose control in 22 studies (55%); (4) appropriate hair removal in 20 studies (50%); (5) mechanical and oral bowel preparation in 18 studies (45%); (6) preoperative bathing with chlorhexidine in 16 studies (40%); (7) standardized postoperative dressing removal and wound care in 16 studies (40%); (8) wound closure protocol including glove with or without gown change and separate instrument tray in 15 studies (38%); (9) use of wound protectors in 11 studies (28%); (10) use of supplemental oxygen postoperatively in 7 studies (18%); (11) cutaneous antisepsis with a solution containing chlorhexidine gluconate and isopropyl alcohol in 6 studies (15%); (12) preoperative smoking cessation in 4 studies (10%); (13) wearing appropriate surgical attire, restricted to the operating room (OR) in 2 studies (5%). Other interventions included minimizing OR traffic,Reference Connolly, Foppa, Kazi, Denoya and Bergamaschi19,Reference Crolla, van der Laan, Veen, Hendriks, van Schendel and Kluytmans20,Reference Elia-Guedea, Cordoba-Diaz de Laspra, Echazarreta-Gallego, Valero-Lazaro, Ramirez-Rodriguez and Aguilella-Diago22,Reference Keenan, Speicher and Nussbaum46,Reference Keenan, Speicher, Thacker, Walter, Kuchibhatla and Mantyh51 peritoneal lavage with antibiotics,Reference Ruiz-Tovar, Llavero, Morales and Gamallo14,Reference Ohman, Wan and Guthrie43 wound irrigation with antibiotic solutionReference Albert, Bataller and Masroor15,Reference Hewitt, Tannouri and Burkhart26 or with saline before closure,Reference Benlice16,Reference Gorgun, Rencuzogullari and Ozben23,Reference Lutfiyya, Parsons and Breen29,Reference Perez-Blanco, Garcia-Olmo, Maseda-Garrido, Najera-Santos and Garcia-Caballero31,Reference Yamamoto, Morimoto and Kita40 postoperative daily chlorhexidine showers,Reference Cima, Dankbar and Lovely18,Reference DeHaas, Aufderheide, Gano, Weigandt, Ries and Faust21,Reference Russell, Chung and Riad32 and daily wound cleaning with chlorhexidine.Reference Russell, Chung and Riad32,Reference Keenan, Speicher and Nussbaum46,Reference Keenan, Speicher, Thacker, Walter, Kuchibhatla and Mantyh51
Adherence to colorectal SSI prevention bundles
Adherence to colorectal SSI prevention bundles was reported in 28 studies (70%). Composite adherence rates to the entire bundle, reported in 7 studies (25%), ranged from 19% in a 9-intervention bundleReference Tanner, Kiernan and Hilliam33 to 92% in a 3-intervention bundle.Reference Berenguer, Ochsner, Lord and Senkowski38 In terms of adherence to individual bundle interventions, several studies reported adherence rates ≥90% to 1 or more Surgical Care Improvement Project (SCIP) measures: correct preoperative antibiotic prophylaxis, hair removal by clippers, normothermia maintenance, with or without preoperative glycemic control.Reference Anthony, Murray and Sum-Ping12,Reference Kwaan, Weight and Carda13,Reference Cima, Dankbar and Lovely18,Reference Crolla, van der Laan, Veen, Hendriks, van Schendel and Kluytmans20,Reference Hedrick, Heckman, Smith, Sawyer, Friel and Foley24,Reference Hewitt, Tannouri and Burkhart26,Reference Pastor, Artinyan, Varma, Kim, Gibbs and Garcia-Aguilar30,Reference Weiser, Gonen and Usiak35,Reference Wick, Galante and Hobson36,Reference Berenguer, Ochsner, Lord and Senkowski38,Reference Forbes, Stephen and Harper41,Reference Keenan, Speicher and Nussbaum46,Reference Tillman, Wehbe-Janek, Hodges, Smythe and Papaconstantinou49,Reference Keenan, Speicher, Thacker, Walter, Kuchibhatla and Mantyh51 In contrast, other studies reported lower adherence rates to these interventions, with antibiotic prophylaxis adherence ranging from 45% to 85%,Reference Albert, Bataller and Masroor15,Reference Benlice16,Reference Perez-Blanco, Garcia-Olmo, Maseda-Garrido, Najera-Santos and Garcia-Caballero31,Reference Tanner, Kiernan and Hilliam33,Reference Mammo, Peeples, Grodsky, Honaker and Wasvary42,Reference Bert, Giacomelli and Amprino52 and normothermia ranging from 33% to 77%.Reference Albert, Bataller and Masroor15,Reference Hedrick, Turrentine and Smith25,Reference Liau, Aung and Chua28,Reference Perez-Blanco, Garcia-Olmo, Maseda-Garrido, Najera-Santos and Garcia-Caballero31,Reference Tanner, Kiernan and Hilliam33 Interventions related to surgical wound closure (ie, glove with or without gown change, separate instrument tray, redraping, and/or wound lavage) had adherence rates ≥85% in several studies.Reference Gorgun, Rencuzogullari and Ozben23,Reference Hewitt, Tannouri and Burkhart26,Reference Ghuman, Chan, Karimuddin, Brown, Raval and Phang45 Adherence to preoperative bathing with chlorhexidine and/or use of chlorhexidine-impregnated wipes ranged between 89% and 92%.Reference Benlice16,Reference Bert, Giacomelli and Amprino52
Three studies reported SSI incidence by bundle adherence: Jaffe et alReference Jaffe, Meka and Semaan27 observed an overall SSI rate of 0.9% versus 16% when there was adherence to all 6 bundle elements versus only 0–2 bundle elements, respectively. Vu et alReference Vu, Collins and Seese34 reported an overall SSI rate of 4.5% versus 6.5% in the highest versus lowest adherence quartile, respectively (P = .049). Waits et alReference Waits, Fritze and Banerjee50 reported an overall SSI rate of 2.5% for adherence to all 6 bundle interventions versus 17.5% for adherence to only 1 bundle intervention.Reference Waits, Fritze and Banerjee50
Implementation strategies for colorectal SSI prevention bundles
The following implementation strategies were most commonly used: (1) multidisciplinary collaborative team or steering committee, led by a colorectal surgery champion, with members from surgery, anesthesia, quality improvement, infection control, infectious disease, pharmacy, hospital administratorsReference Albert, Bataller and Masroor15–Reference Connolly, Foppa, Kazi, Denoya and Bergamaschi19,Reference DeHaas, Aufderheide, Gano, Weigandt, Ries and Faust21,Reference Hedrick, Heckman, Smith, Sawyer, Friel and Foley24,Reference Pastor, Artinyan, Varma, Kim, Gibbs and Garcia-Aguilar30,Reference Vu, Collins and Seese34,Reference Weiser, Gonen and Usiak35,Reference Forbes, Stephen and Harper41,Reference Keenan, Speicher and Nussbaum46,Reference Tillman, Wehbe-Janek, Hodges, Smythe and Papaconstantinou49 ; (2) surgical leadership spearheading bundle implementationReference Benlice16,Reference Cima, Dankbar and Lovely18,Reference Connolly, Foppa, Kazi, Denoya and Bergamaschi19,Reference Hewitt, Tannouri and Burkhart26,Reference Berenguer, Ochsner, Lord and Senkowski38,Reference Keenan, Speicher and Nussbaum46 ; (3) hospital administration leadership support for process enforcement and resource allocationReference Crolla, van der Laan, Veen, Hendriks, van Schendel and Kluytmans20,Reference Wick, Hobson and Bennett37 ; (4) educational meetings with relevant frontline cliniciansReference Connolly, Foppa, Kazi, Denoya and Bergamaschi19,Reference Hedrick, Heckman, Smith, Sawyer, Friel and Foley24,Reference Hewitt, Tannouri and Burkhart26,Reference Wick, Hobson and Bennett37,Reference Forbes, Stephen and Harper41,Reference Tillman, Wehbe-Janek, Hodges, Smythe and Papaconstantinou49 ; (5) assignment of responsibility and accountability for key bundle interventions to most relevant cliniciansReference Hedrick, Heckman, Smith, Sawyer, Friel and Foley24,Reference Hedrick, Turrentine and Smith25,Reference Lutfiyya, Parsons and Breen29 ; (6) use of checklist to record process measures and improve adherence with prevention bundleReference Bull, Wilson and Worth17,Reference Connolly, Foppa, Kazi, Denoya and Bergamaschi19,Reference Hedrick, Turrentine and Smith25,Reference Hewitt, Tannouri and Burkhart26,Reference Wick, Hobson and Bennett37,Reference Reames, Krell, Campbell and Dimick39 ; (7) use of electronic order sets and automatic reminders to facilitate bundle related workflowsReference Cima, Dankbar and Lovely18,Reference Hedrick, Heckman, Smith, Sawyer, Friel and Foley24,Reference Russell, Chung and Riad32,Reference Wick, Galante and Hobson36,Reference Keenan, Speicher and Nussbaum46 ; (8) standardization of clinical practices and protocols across all operating unitsReference Hedrick, Heckman, Smith, Sawyer, Friel and Foley24,Reference Hedrick, Turrentine and Smith25 ; (9) performance feedback to staff and clinicians on a routine basisReference Cima, Dankbar and Lovely18,Reference Crolla, van der Laan, Veen, Hendriks, van Schendel and Kluytmans20,Reference Hedrick, Heckman, Smith, Sawyer, Friel and Foley24,Reference Hedrick, Turrentine and Smith25,Reference Lutfiyya, Parsons and Breen29,Reference Russell, Chung and Riad32,Reference Vu, Collins and Seese34,Reference Wick, Galante and Hobson36,Reference Forbes, Stephen and Harper41,Reference Keenan, Speicher and Nussbaum46 ; and (10) overall promotion of culture of safety and openness to change.Reference Connolly, Foppa, Kazi, Denoya and Bergamaschi19,Reference Crolla, van der Laan, Veen, Hendriks, van Schendel and Kluytmans20,Reference Wick, Galante and Hobson36,Reference Reames, Krell, Campbell and Dimick39
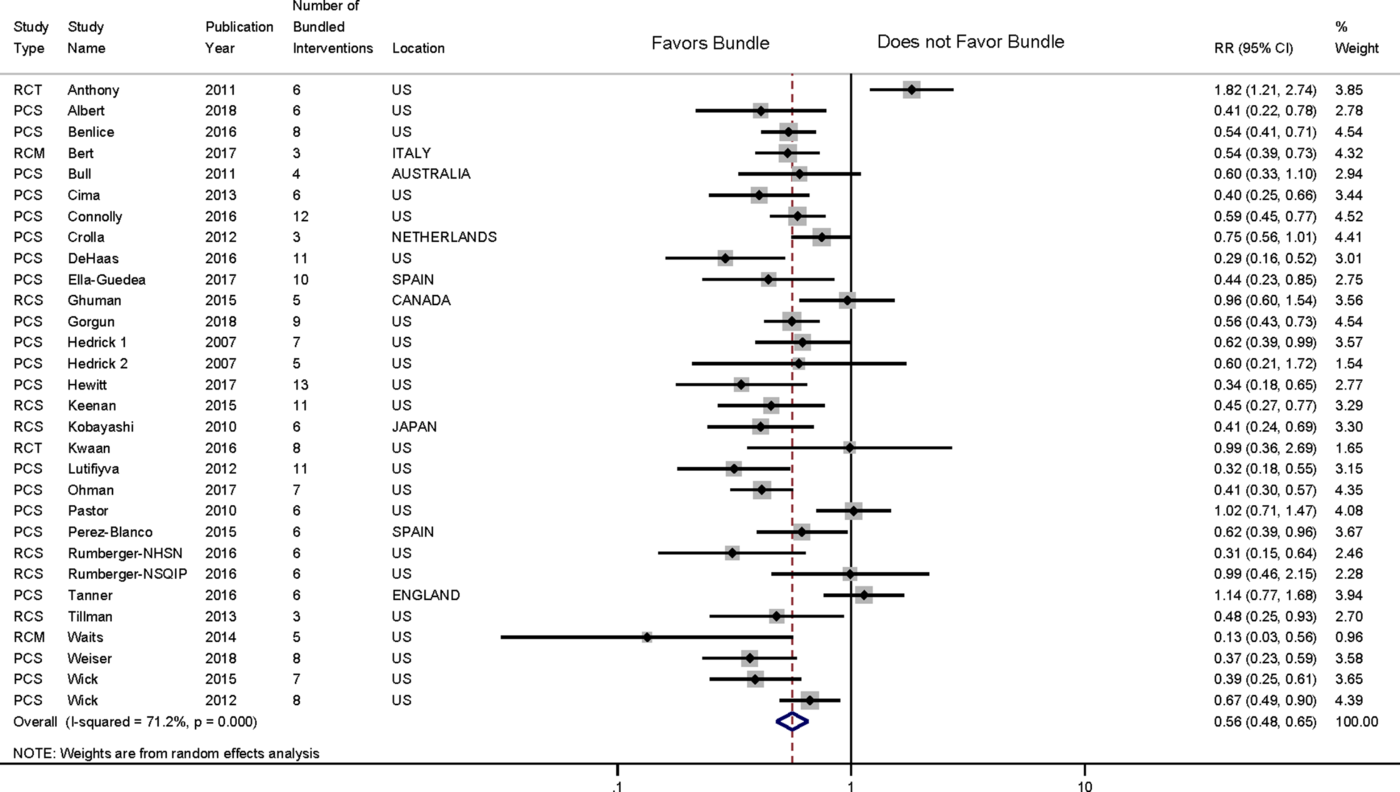
Effect of colorectal bundles on incidence of overall SSI (meta-analysis)
We included 30 studies that reported overall SSI incidence outcomes in our meta-analysis (Fig. 2 and Supplemental Fig. S4 online). We analyzed SSI outcomes separately for the single study that reported SSI outcomes by 2 different surveillance methodologies (NHSN and NSQIP).Reference Rumberger, Vittetoe, Cathey, Bennett, Heidel and Daley48 Cumulatively, there were 894 SSI among the 10,627 patients in the study arm versus 1,561 SSI among the 10,074 patients in the control arm. We found a statistically significant pooled SSI RR of 0.56 (95% CI, 0.48–0.65), with 71.2% heterogeneity in our random effects model. There was no significant publication bias according to the Egger test (−0.48; P = .60) and Begg’s Kendall score (Q = −23; P = .69). Given the high statistical heterogeneity, we analyzed the bundle effect on SSI incidence over 2 periods: 2000–2015 and 2016–2018. We found a pooled SSI RR of 0.60 (95% CI, 0.49–0.70) with 73.7% heterogeneity for studies published during 2000–2015, and an RR of 0.48 (95% CI, 0.41–0.57) with 0.0% heterogeneity for 2016–2018. Cumulative effects analysis showed that all studies included showed a protective SSI effect, except for the study by Anthony et al,Reference Anthony, Murray and Sum-Ping12 which showed an SSI RR of 2.49 (Supplemental Fig. S5 online).

Fig. 2. Forest plot showing pooled effect of colorectal bundles on the incidence of overall surgical site infection. Note. RCT, randomized controlled trial; PCS, prospective cohort, single center; PCM, prospective cohort, multicenter; RCS, retrospective cohort, single center; RCM, retrospective cohort, multicenter.
Effect of colorectal bundles on incidence of superficial SSI (meta-analysis)
We included 21 studies that reported superficial SSI incidence outcomes in our meta-analysis (Supplemental Fig. S3 online). There were 808 SSIs among the 19,984 patients in the study arm, versus 1,094 SSIs among the 19,479 patients in the control arm. We found a statistically significant pooled SSI RR of 0.56 (95% CI, 0.42–0.75), with 83.4% heterogeneity in our random-effects model. Publication bias was present according to the Egger test (−2.1; P = .009), but not according to Begg’s Kendall score (Q = −32; P = .34).
Effect of colorectal bundles on incidence of deep SSI (meta-analysis)
We included 11 studies that reported deep SSI incidence outcomes in our meta-analysis. (Supplemental Fig. S2 online). There were 36 SSIs among the 3,365 patients in the study arm, versus 78 SSIs among the 3,787 patients in the control arm. In our fixed-effects model, we calculated a statistically significant pooled SSI RR of 0.67 (95% CI, 0.45–0.98), with 0.0% heterogeneity. There was no significant publication bias according to the Egger test (−0.332.1; P = .059) and Begg’s Kendall score (Q = −4; P = .71)
Effect of colorectal bundles on incidence of organ-space SSI (meta-analysis)
We included 17 studies that reported organ-space SSI incidence outcomes into the meta-analysis. There were 203 SSIs among the 6,115 patients in the study arm, versus 344 SSIs among the 5,980 patients in the control arm. In our random-effects model, we found a statistically significant pooled SSI RR of 0.63 (95% CI, 0.49–0.81), with 44.5% heterogeneity (Supplemental Fig. S1). There was no significant publication bias according to the Egger test (2.1; P = .059) and Begg’s Kendall score (Q = 30; P = .23).
Effect of bundle size on SSI (meta-regression)
In our meta-regression analysis, we found that bundle size was statistically significantly related to reduction of overall SSI in our meta-regression model (Table 2). The highest SSI reduction (59%) was associated with the largest bundle size, corresponding to colorectal SSI prevention bundles whose composition included ≥11 ACS/SIS guideline-recommended interventions.
Table 2. Meta Regression Models Examining Bundle Size Categories and Reduction of Surgical Site Infections

Note. Het I2, test of heterogeneity; RR, relative risk; CI, confidence interval. Bundle size represents number guideline-recommended interventions included in each bundle.
Effect of individual bundle interventions on SSI
The following individual colorectal bundle interventions significantly correlated with SSI incidence: (1) mechanical bowel preparation combined with oral antibiotics (R = −0.68; P = .003) and (2) preoperative chlorhexidine showers (R = −0.49; P = .04) for organ-space SSI.
The following bundle interventions correlated with highest superficial SSI reductions: (1) wound closure protocols, including separate instrument trays and glove with or without gown change prior to surgical wound closure (R = −0.55; P = .009), and (2) standardized postoperative wound dressing change at 48 hours (R = −0.59; P = .005)
Discussion
Bundles containing ≥11 ACS/SIS recommended interventions had the lowest SSI risk. These large size bundles included ACS/SIS recommended interventions that were already standard of care in addition to newer interventions. The explicit presence of all these elements within prevention bundles emphasizes the significance of maintaining adherence to well-established standards of care while also implementing new elements into clinical practice. The listing of ≥20 different interventions for SSI prevention in most clinical guidelinesReference Ban, Minei and Laronga5,53,54 further underscores this point, highlighting the unique complexity of SSI prevention.
Achieving optimal composite adherence to such complex bundles remains extremely challenging. Studies such as those by Tanner et al,Reference Tanner, Kiernan and Hilliam33 in which composite adherence to a 9-element colorectal bundle was only 19% and SSIs were not reduced, illustrate how difficult it is to successfully translate complex bundled interventions into clinical practice. The implementation strategies compiled in our systematic review fell into the “Four Es” model recommended for preventing healthcare-associated infections: engagement, education, execution, and evaluation.Reference Septimus, Yokoe, Weinstein, Perl, Maragakis and Berenholtz55,Reference Ariyo, Zayed and Riese56 These strategies, in conjunction with efforts that promote a culture of safety and openness to change, can aid in increasing adherence. Further research should focus on how to implement complex evidence-based bundles with high adherence in the clinical setting.
The composition of the “ideal” SSI prevention bundle in colorectal surgery remains unclear. The meta-analysis by Zywot et al,Reference Zywot, Lau, Stephen Fletcher and Paul57 published in 2017, identified colorectal bundles consisting of sterile closure trays, combined oral and mechanical bowel preparation, and preclosure glove change as having greater overall SSI risk reductions. Our meta-analysis, which included 3 studies subsequently published in 2018, used a different methodology to assess the impact of individual bundle components on SSI. We found that 2 additional elements, preoperative bathing with chlorhexidine and standardized postoperative wound dressing changes at 48 hours, also significantly correlated with SSI reductions. We also confirmed the 3 bundle elements found in the previous study, suggesting that their inclusion in prevention bundles is important. Other guideline-recommended interventions with high-quality evidence, such as preoperative antibiotic prophylaxis, perioperative glycemic control, normothermia maintenance, and cutaneous antisepsis with an alcohol-based antiseptic agent,Reference Berrios-Torres, Umscheid and Bratzler58 were all part of the large bundles with the highest SSI reductions.Reference Connolly, Foppa, Kazi, Denoya and Bergamaschi19,Reference DeHaas, Aufderheide, Gano, Weigandt, Ries and Faust21,Reference Hewitt, Tannouri and Burkhart26,Reference Lutfiyya, Parsons and Breen29,Reference Keenan, Speicher and Nussbaum46,Reference Keenan, Speicher, Thacker, Walter, Kuchibhatla and Mantyh51 Given the unique characteristics of hospitals, performing different types of colorectal surgeries with variable preoperative risk, it is likely that bundle heterogeneity will continue in clinical practice. Further research should address how preventive bundles are best tailored to each clinical context.
Although we found that colorectal bundles are effective in preventing SSIs, our study has several limitations. First, bundle composition varied significantly across studies. Interventions that are not currently recommended by the ACS/SIS, such as peritoneal cavity or surgical wound irrigation with antibiotic solution or saline, and postoperative daily wound cleaning with chlorhexidine, were also included. Although these measures are not supported by current clinical practice guidelinesReference Berrios-Torres, Umscheid and Bratzler58,Reference Pouralizadeh, Khankeh, Ebadi and Dalvandi59 due to lack of individual SSI effect, a potential synergistic bundle effect remains possible, although it is difficult to quantify. Second, SSI incidence was assessed using different surveillance methodologies, which may have led to over- or underestimation of SSI outcomes in some studies. For studies conducted in the United States, the 2 main surveillance systems—National Surgical Quality Improvement Program (NSQIP) and National Healthcare Safety Network (NHSN)—have shown occasional discrepancies in colon SSI rates,Reference Ju, Ko, Hall, Bosk, Bilimoria and Wick60,Reference Bordeianou, Cauley and Antonelli61 as demonstrated by Rumberger et alReference Rumberger, Vittetoe, Cathey, Bennett, Heidel and Daley48 (included in this review). Reconciling methodological differences in risk adjustment between different national surveillance systems would increase the accuracy of reported SSI incidence. Third, the greatest SSI reduction benefit in our meta-analysis came from observational trials, many of which were at medium to high risk of bias. Only 5 studies were multicentered, and only 3 studies were prospective in nature. Only 3 randomized controlled trials were included, all conducted at a single center, and only 1 of these studies showed bundle-associated SSI reduction, after elective laparoscopic colorectal cancer surgery.Reference Ruiz-Tovar, Llavero, Morales and Gamallo14 The study by Anthony et alReference Anthony, Murray and Sum-Ping12 showed a large increase in SSI, in part due to restricting intraoperative fluids in the intervention group and to assigning mechanical and oral bowel preparation to their control group. The third randomized-controlled study was underpowered for the lower-than-expected SSI rate observed in the trial and was stopped midtrial.Reference Kwaan, Weight and Carda13 Thus, the need for well-designed, high-quality prospective studies focusing on bundle efficacy remains.
In conclusion, colorectal bundles that include ≥11 guideline-recommended interventions are important tools in SSI prevention. Their efficacy and implementation, tailored to the clinical context, should be tested in high-quality, well-designed prospective trials.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2020.112
Acknowledgments
Financial support
This work was supported by the Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin.
Potential conflicts of interest
All authors report no conflicts of interest relevant to this article.