Candida auris is a globally emerging pathogen that is often resistant to multiple antifungal agents.Reference Vallabhaneni, Kallen and Tsay 1 – Reference Larkin, Hager and Chandra 3 In several reports, C. auris has been recovered from the hospital environment, suggesting that contaminated surfaces may be a source of transmission.Reference Vallabhaneni, Kallen and Tsay 1 , Reference Schelenz, Hagen and Rhodes 2 The Centers for Disease Control and Prevention (CDC) has therefore recommended daily and postdischarge disinfection of surfaces in rooms of patients with C. auris infection. 4 Moreover, the use of an Environmental Protection Agency (EPA)–registered, hospital-grade disinfectant effective against Clostridium difficile spores was specified. 4 However, nonsporicidal disinfectants are widely used and many have an EPA-registered antifungal claim. 5 No hospital disinfectants are registered for use specifically against C. auris, and it is not known whether C. auris is less susceptible to disinfectants than other Candida species.
METHODS
We evaluated the efficacy of 9 commercial disinfectants and the common household disinfectant white distilled vinegar (>5% acetic acid, pH 2.0) against C. auris, C. albicans, and C. glabrata. For comparison, 3 strains of methicillin-resistant Staphylococcus aureus (MRSA) were tested. The 5 C. auris strains have been described previouslyReference Larkin, Hager and Chandra 3 , 6 ; the strains included 3 multidrug-resistant clinical isolates (MRL#31102 and #31103 and CBS#12373) and 2 drug-susceptible isolates (CBS#10913 and CDC strain AR-BANK#0381). The 4 C. albicans strains included American Type Culture Collection (ATCC) strains SC#5314 and #10231 and 2 clinical strains from the Center for Medical Mycology, Cleveland, Ohio (MRL#32249 and #32708). The C. glabrata clinical strains were MRL#31820, #34870, and #9547. The 3 MRSA strains included ATCC strain 43,300 and 2 clinical isolates.
The disinfectants and their contact times are shown in Table 1. For the commercial products, the contact times were based on the manufacturer’s recommendations except for Clorox Healthcare hydrogen peroxide cleaner disinfectant (ie, a 1-minute exposure was used rather than the 3 minutes recommended for C. albicans based on preliminary experiments that demonstrated excellent activity with the shorter exposure time). Testing of disinfectant efficacy was conducted according to the American Society for Testing and Materials (ASTM) Standard quantitative carrier disk test method (ASTM E2197-11). 7 To simulate the presence of organic material, 5% fetal calf serum (Remel, Lenexa, KS) was added to the inoculum. The steel-disk carriers were inoculated with ~106 colony-forming units (CFUs) of washed Candida species or MRSA from an overnight culture; the test was not considered valid unless a minimum of 105 CFU was recovered from control carriers. After disinfectant exposure, carriers were neutralized with Dey-Engley neutralizer (Remel), except for Oxivir Tb (Diversey, Charlotte, NC) and Clorox Healthcare hydrogen peroxide cleaner disinfectant (Clorox, Oakland, CA), which were neutralized with 0.1% Tween 80 and 3% glycine in 0.85% NaCl. Serial dilutions were plated onto Sabouraud dextrose agar (Becton-Dickinson, Sparks, MD) and incubated at 37°C for 72 hours. Log reductions were calculated by subtracting viable organisms recovered after disinfectant exposure versus deionized water controls. Experiments were performed in triplicate.
TABLE 1 Characteristics of the Disinfectants Tested
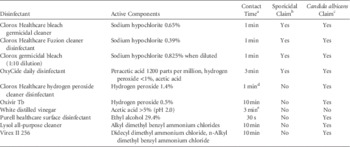
a Contact time for disinfectants based on manufacturers’ recommendations for Candida albicans unless otherwise specified.
b Environmental Protection Agency–registered claim against Clostridium difficile spores.
c Environmental Protection Agency–registered claim against Candida albicans.
d A 1-minute exposure was used, but the claim is based on a 3-minute exposure.
e There is no established contact time for vinegar.
To determine whether different test methods might yield different results for a quaternary ammonium compound, we compared reductions of the Candida species and MRSA for Virex II 256 using ASTM E-2197-11 and the AOAC (Association of Official Agricultural Chemists) Official Method 961.02 for Germicidal Spray Products. 8 For this comparison, we included tests with a lower inoculum resulting in 104 CFU recovered from control carriers. The rationale for testing the reduced inoculum was that the EPA product performance test guideline OCSPP 810.2200 for disinfectants with fungicidal claims recommends that carriers have a concentration of 104 to 105 CFU per carrier. 9 In the AOAC method, glass microscope slides are used as carriers and the inoculum is spread to cover approximately 1 square inch. For all experiments, log reductions were compared for different disinfectants using linear modeling with post hoc Tukey’s adjustment for multiple comparisons. Data were analyzed using SPSS statistical software version 10.0 (SPSS, Chicago, IL).
RESULTS
Figure 1 shows the mean reductions for the disinfectants using the ASTM method. For MRSA, each of the disinfectants reduced recovery by ~6 log10 CFU. The data for each of the Candida species was pooled because there were no significant differences in reductions for different strains. The 3 bleach disinfectants, OxyCide, Clorox Healthcare hydrogen peroxide cleaner disinfectant, and Oxivir TB consistently reduced the Candida species by ≥5 log10 CFU, and there were no significant differences in reductions for the Candida species and MRSA (P>.05). Vinegar, Purell Healthcare surface disinfectant (Gojo, Akron, OH), and the 2 quaternary ammonium disinfectants were significantly less effective against Candida species than against MRSA (P≤.02). There were no significant differences in efficacy against C. auris versus the other Candida species.
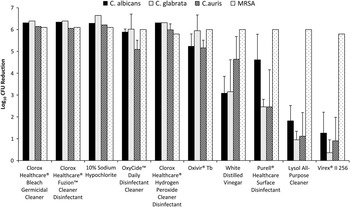
FIGURE 1 Mean log reductions for each of the disinfectants against the 3 Candida species and MRSA using the American Society for Testing and Materials (ASTM) Standard Quantitative Carrier Disk Test Method (ASTM E-2197-02). 7 Log reductions were calculated by subtracting viable organisms recovered after exposure to the disinfectants versus deionized water controls. Vinegar, Purell Healthcare Surface Disinfectant, and the 2 quaternary ammonium disinfectants were significantly less effective against the Candida species than against MRSA (P≤.02). Error bars show standard error. MRSA, methicillin-resistant Staphylococcus aureus.
Compared to the ASTM method, the AOAC method resulted in significantly greater measured log reductions (mean±SE) for Virex II 256 against C. albicans (3.3±0.4 versus 0.5±0.4), C. glabrata (3.8±0.4 vs 0.4±0.2), and C. auris (2.2±.2 vs 0.7±0.3) (P<.05 for each comparison), but not MRSA (ie, ~6 log10CFU reductions with both methods), when the inoculum was 106 CFUs per carrier (P<.05 for each comparison).
For the ASTM E2197-11 method, reducing the inoculum such that 104 CFUs were recovered from the control carriers significantly enhanced the measured reduction in Candida species achieved by Virex II 256 (~3 vs 0.5 to 2 log reduction; P<.01). For the AOAC method, a similar reduction in the inoculum resulted in 4 log reductions in each of the Candida species (data not shown).
DISCUSSION
Many disinfectants have an EPA-registered fungal claim based on efficacy against C. albicans or Trichophyton mentagrophytes. 5 However, interim recommendations from the CDC have specified that an EPA-registered hospital-grade disinfectant effective against C. difficile spores be used for C. auris. 4 Our results provide support for that recommendation but also demonstrate that nonsporicidal improved hydrogen peroxide disinfectants are highly active against Candida species, including C. auris. There was no evidence that C. auris has greater resistance to disinfectants than do other Candida species.
The relatively poor activity of the quaternary ammonium disinfectants against all Candida species is concerning because these agents are widely used. In contrast to MRSA, we found that differences in test methods may markedly impact the measured efficacy of quaternary ammonium disinfectants against Candida species. Our results suggest that factors such as use of a smaller organism inoculum and spreading of the inoculum may enhance the measured reductions. In that regard, it should be noted that the EPA has recently released interim guidance for testing the efficacy of liquid antimicrobials against C. auris 6 ; the recommended methods are similar to the ASTM method used in our study, and both methods yield similar results (unpublished data).
It is possible that the environment may be an underappreciated reservoir for the spread of Candida species other than C. auris. In a recent study, we demonstrated that C. auris and other Candida species have a similar propensity to survive on surfaces, and Candida species were frequently recovered from the hospital environment, particularly from moist surfaces.Reference Piedrahita, Cadnum, Jencson, Shaikh, Ghannoum and Donskey 10 Moreover, previous studies have implicated the environment as a potential source for transmission of Candida species.Reference Pfaller 11
Our study has some limitations. We studied only 2 quaternary ammonium disinfectants and cannot exclude the possibility that other quaternary ammonium compounds may have greater activity. We did not assess the efficacy of the disinfectants on hospital surfaces. It is possible that quaternary ammonium compounds may exhibit greater efficacy on real-world surfaces if smaller numbers of Candida species are present.
ACKNOWLEDGMENTS
Financial support: This work was funded by a Merit Review grant (grant no. BX002944-01A1) from the Department of Veterans Affairs to C.J.D.
Potential conflicts of interest: C.J.D. has received research grants from Merck, GOJO, Altapure, EcoLab, and Clorox and serves on advisory boards for 3M and Synthetic Biologics. All other authors report no conflicts of interest relevant to this article.




