Introduction
Alzheimer's disease (AD) pathology likely precedes the development of clinical manifestations by several years or decades (Braak and Braak, Reference Braak and Braak1991; Shaw et al., Reference Shaw, Korecka, Clark, Lee and Trojanowski2007). In order to study preclinical AD, it is critical to use in vivo biomarkers such as amyloid imaging (Dubois et al., Reference Dubois2007; Sperling et al., Reference Sperling2011). The amyloid cascade hypothesis posits that the accumulation of amyloid β-peptide in the brain is a necessary component of the pathophysiology of AD (Hardy and Selkoe, Reference Hardy and Selkoe2002; Choi et al., Reference Choi2014). About 30% of cognitively normal persons above the age of 70 years, 60% of patients with mild cognitive impairment (MCI) and 85% of clinically diagnosed AD patients are amyloid positive (Jack et al., Reference Jack, Barrio and Kepe2013). However, studies have found an association between amyloid deposition with a faster cognitive decline in both cognitively normal elderly (Morris et al., Reference Morris2009; Chetelat et al., Reference Chetelat2012; Knopman et al., Reference Knopman2012; Petersen et al., Reference Petersen2016) and MCI subjects (Jack et al., Reference Jack2010; Jagust et al., Reference Jagust2010). A growing body of research has shown that depressive and anxiety symptoms may increase the risk of cognitive decline (Geda et al., Reference Geda2006, Reference Geda2008, Reference Geda2014; Pink et al., Reference Pink2015). Furthermore, we and others have reported that depressive (Donovan et al., Reference Donovan2015; Krell-Roesch et al., Reference Krell-Roesch2016) and anxiety symptoms (Pink et al., Reference Pink2017) are associated with neuroimaging biomarkers of AD, specifically FDG-PET and cortical thickness as measured by MRI, among cognitively normal elderly participants. To date, only few studies have investigated the association between amyloid imaging and depression (Butters et al., Reference Butters2008; Chung et al., Reference Chung2016; Holmes et al., Reference Holmes2016; Harrington et al., Reference Harrington2017) or anxiety (Pietrzak et al., Reference Pietrzak2014; Pietrzak et al., Reference Pietrzak2015; Holmes et al., Reference Holmes2016). We therefore sought to examine the association between cortical amyloid deposition, as measured by Pittsburgh compound B positron emission tomography (PiB-PET), depressive and anxiety symptoms in a population-based sample of cognitively normal participants. Given the current discussion in the literature on whether prodromal neuropsychiatric symptoms should be assessed on a continuum rather than relying on arbitrary cut-offs (Altman and Royston, Reference Altman and Royston2006; Bjelland et al., Reference Bjelland, Lie, Dahl, Mykletun, Stordal and Kraemer2009; Laborde-Lahoz et al., Reference Laborde-Lahoz2014), we used both continuous and categorical approaches when assessing depressive and anxiety symptoms. We hypothesized that both anxiety and depressive symptoms would be associated with higher cortical amyloid deposition as measured by PiB-PET in our sample of community-dwelling cognitively normal elderly persons.
Methods
Setting. This study was conducted in the setting of the population-based Mayo Clinic Study of Aging (MCSA). Details of the design and conduct of the MCSA have been reported elsewhere (Roberts et al., Reference Roberts2008). Briefly, the MCSA is an ongoing population-based study of cognitive aging in Olmsted County, Minnesota. Participants who were ≥ 60 years old, had undergone brain amyloid imaging and completed both depressive and anxiety symptom assessments were included in this study. The study was conducted with the approval of the institutional review boards of the Mayo Clinic and Olmsted Medical Center in Rochester, Minnesota and with the written informed consent of each participant.
Cognitive evaluation. Participants underwent extensive evaluation including neurological examination, psychometric testing and risk factor assessment as described in detail elsewhere (Roberts et al., Reference Roberts2008). An expert consensus panel consisting of physicians, neuropsychologists, and nurses or study coordinators reviewed all pertinent data and made the diagnosis of normal cognition, MCI, or dementia. Individuals were considered cognitively normal at baseline according to published normative data developed on this community (Ivnik et al., Reference Ivnik1992a,Reference Ivnik1992b, Reference Ivnik1992c, Reference Ivnik1992d; Malec et al., Reference Malec1992). For MCI, the following revised Mayo Clinic criteria for MCI (Petersen, Reference Petersen2004; Winblad et al., Reference Winblad2004) were used: (1) cognitive concern expressed by a physician, informant, participant, or nurse; (2) impairment in one or more cognitive domains (memory, language, visuospatial skills, or executive functions); (3) essentially normal functional activities; and (4) absence of dementia. Participants with MCI had a Clinical Dementia Rating Scale score of 0 or 0.5; however, the final diagnosis of MCI was based on all available data. Participants with MCI or dementia were excluded from this study.
Measurement of depressive and anxiety symptoms. Depressive symptoms were measured using a validated, self-administered Beck Depressive Inventory-II (BDI) which is a sensitive instrument in elderly subjects (Beck et al., Reference Beck, Steer and Brown1996). Thus, the BDI-II likely captures as many depressive symptoms as possible and we considered it to be an appropriate screening instrument to use in a large scale population-based study. The BDI-II consists of 21 items such as feeling guilty and loss of interest, that are assessed over the last two weeks and are rated in severity on a 4-point Likert scale ranging from 0 to 3; the total score ranges from 0 to 63. Symptoms of anxiety were measured using the Beck Anxiety Inventory (BAI), a validated, self-administered questionnaire (Beck et al., Reference Beck, Epstein, Brown and Steer1988). Like the BDI-II, the BAI also consists of 21 items that are assessed over the last week and measure common symptoms of anxiety, such as nervousness and fear of losing control. The severity of each symptom is rated ranging from 0 to 3 with a total score ranging from 0 to 63.
Neuroimaging of amyloid deposition. We performed amyloid PET imaging using Pittsburgh Compound B tracer. PiB scans, consisting of four 5-minute dynamic frames, were acquired from 40 to 60 minutes after intravenous injection with 292–728 MBq (11C)PiB. Images were analyzed using an in-house, fully automated image processing pipeline, in which image voxel values were extracted from automatically labeled regions of interest propagated from an MRI template. A global amyloid PET standardized uptake value ratio (SUVR) was formed from the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest normalized to the cerebellar grey matter. Participants with an SUVR ≥ 1.40 were classified as having an abnormal PiB-PET (amyloid positive; PiB+) as we have previously validated by autopsy (Murray et al., Reference Murray2015).
Statistical Analysis. We used multi-variable logistic regression analyses to examine the association between BDI and BAI scores with an abnormal PiB-PET scan (PiB+ vs. PiB−, modeled on BAI, BDI) by calculating odds ratios (OR) and 95% confidence intervals (95% CI) after adjusting for age and sex. Furthermore, we conducted linear regression analyses to investigate the association between BDI and BAI scores with elevated amyloid deposition in the brain (PiB amyloid SUVR modeled on BAI, BDI) by computing β estimates, and standard errors of the mean (SE) after adjusting for age and sex. For both regression analyses, we treated both BDI-II and BAI as continuous variables. In addition, we examined BDI-II and BAI as categorical variables with different cut-offs (BDI-II cut-offs: 4 (median number of symptoms in the study population), and 13 (cut-off for clinical symptoms); BAI cut-offs: 2 (median number of symptoms in the study population), and 10 (cut-off for clinical symptoms). Furthermore, we conducted an analysis stratified by four quartiles (with no symptoms being the reference group; score = 0). Statistical testing was done at the conventional 2-tailed alpha level of p < 0.05. All analyses were performed using SAS System, version 9.3 software (SAS Institute, Cary, NC).
Results
Of 1,038 cognitively normal participants, 551 (53.1%) were males and the median age was 73 years (interquartile range, IQR 67, 79). Three hundred and seventy nine participants (37%) were classified as having an abnormal amyloid deposition (PiB+). PiB+ participants were significantly older than PiB− participants (p < 0.001). In addition, 39% of PiB+ participants were APOE ε4 carriers whereas only 19.9% of PiB− participants were APOE ε4 carriers (p < 0.001). There were few participants with clinical depression (N = 63, 6.1%) or clinical anxiety (N = 51, 4.9%), and the median BDI (3; IQR 1, 6) and BAI (1; IQR 0, 4) scores were low. Table 1 shows the demographic characteristics of PiB+, PiB− and total study population. In all regression analyses, the predictor variables (PiB+ vs. PiB− as categorical variables; and PiB amyloid SUVR as continuous variable) were modeled on the outcome variables (BAI and BDI as categorical and continuous variables). The logistic regression analysis, adjusted for age and sex, revealed that both depression (OR = 1.03; 1.00–1.06) and anxiety (OR = 1.04; 1.01–1.08) were associated with higher odds for an abnormal PiB-PET when examined as continuous variables. The association was significant for anxiety (p = 0.022) and marginally significant for depression (p = 0.077). When we categorized BDI-II and BAI, we observed that the odds of being PiB-PET+ were elevated for participants with BDI ≥ 13 which indicates clinical depression as compared to participants with BDI <13 (OR = 1.42; 0.83–2.43). The same pattern was found for participants with BAI ≥ 10 which indicates clinical anxiety as compared to participants with BAI <10 (OR = 1.77; 0.97–3.22). The effect sizes were smaller for the other group comparisons (BDI: ≥ 10 vs. < 10, ≥ 4 vs. < 4; BAI: ≥ 8 vs. < 8, ≥ 2 vs. < 2). None of these associations reached statistical significance. The stratified analysis by quartiles also showed a trend for higher odds with higher scores on BDI and BAI as compared to the reference groups (score = 0; no symptoms), however lacking statistical significance (Table 2). When we examined the association between amyloid deposition as measured by SUVR and depressive and anxiety symptoms (both treated as numeric variables) using linear regression analysis, we observed significant associations between depressive symptoms (β = 0.005, SE = 0.002, p = 0.007) and amyloid deposition as well as between anxiety symptoms (β = 0.006, SE = 0.003, p = 0.027) and amyloid deposition, after adjusting for age and sex (Table 3).
Table 1. Demographics characteristics of study participants
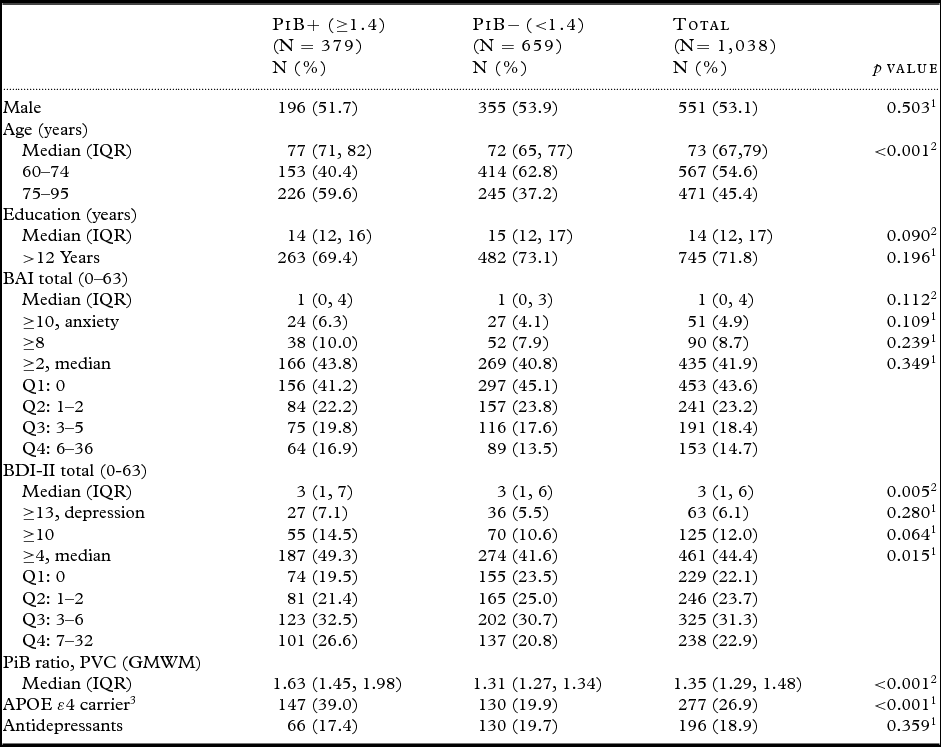
IQR = interquartile range; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; PVC = partial volume correction; GMWM = gray matter-white matter; APOE ε4 = Apolipoprotein ε4.
1 χ 2 test.
2 Wilcoxon rank-sum test.
3 Data missing for seven participants. Antidepressant medication includes SSRI, SNRI, Tetracyclic, Tricyclic, and any other.
Table 2. Logistic regression analysis on the association between amyloid positivity with depressive and anxiety symptoms, adjusted for age and sex
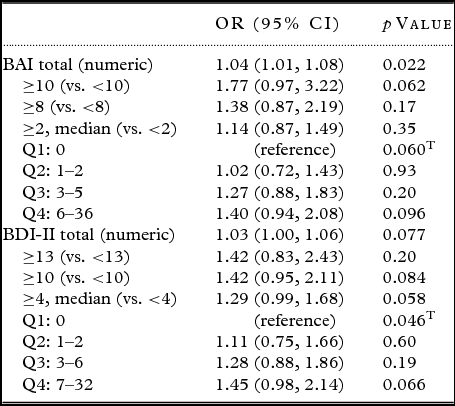
BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; Q = quartile; T = Trend; OR = Odds ratio; 95% CI = 95% confidence interval.
Table 3. Linear regression analysis on the association between amyloid SUVR with depressive and anxiety symptoms, adjusted for age and sex
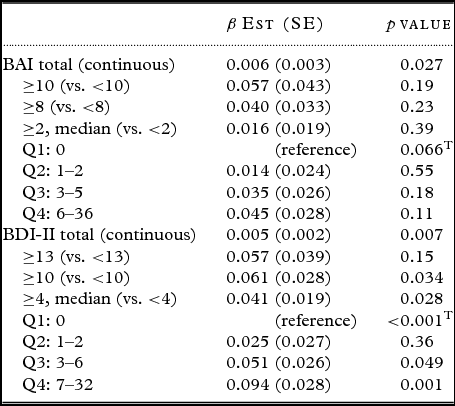
BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; Q = quartile; T = Trend; β Est = β estimate; SE = standard error of the mean.
Discussion
Here, we report the cross-sectional association between amyloid deposition as measured by PiB-PET and depressive and anxiety symptoms among community-dwelling persons free of MCI or dementia. We and others have examined the role of neuropsychiatric symptoms in predicting the progression from CN status to incident MCI (Geda et al., Reference Geda2014). Furthermore, an expert panel of the Alzheimer's Association (ISTAART Neuropsychiatric Symptoms Professional Interest Area) has also called for the systematic investigation of emotional behavior among cognitively normal persons (Ismail et al., Reference Ismail2016). When we treated depressive and anxiety symptoms as continuous variables, we observed an association in the logistic regression analysis between anxiety and an abnormal PiB-PET as well as depression and an abnormal PiB-PET, even though the latter was not significant. When we assessed depressive and anxiety symptoms as categorical variables, we observed higher effect sizes with higher scores on BDI and BAI. The highest effect sizes were found when we used the clinical cut-offs (BDI ≥ 13, BAI ≥ 10). The lack of statistical significance for this analysis may be due to low power as only a small percentage of the PiB+ study population reported depression (7.1%) and anxiety symptoms (6.3%). Similarly, when the associations were stratified by quartiles of BDI-II and BAI symptoms, we observed the same trend: participants in quartiles reflecting higher scores of depressive and anxiety symptoms had higher odds for an abnormal PiB-PET. However, none of these trends reached statistical significance. The results of the linear regression analysis were in line with the logistic regression analysis and revealed a significant association between depressive and anxiety symptoms with amyloid deposition. We also conducted logistic and linear regression analyses stratified by sex and APOE ε4 carrier status in order to examine potential effects on the association between depression, anxiety and PiB-PET positivity. Neither sex nor APOE ε4 carrier status significantly altered the results (data not shown). To date, only few studies have examined the cross-sectional association between amyloid deposition and neuropsychiatric symptoms in presymptomatic AD. For instance, in line with our findings, a systematic review observed significant differences in beta amyloid levels between depressed and non-depressed older adults in the majority of included studies (Harrington et al., Reference Harrington, Lim, Gould and Maruff2015). In addition, the review concluded that individuals with depression showed higher amyloid binding on PET; however, the review only included five studies that measured beta amyloid using PET neuroimaging (two using PiB, three using other ligands) (Harrington et al., Reference Harrington, Lim, Gould and Maruff2015). Investigators from the Australian AIBL study recently reported that sex moderated the relationship between amyloid deposition, APOE genotype, and anxiety and depressive symptoms among 423 cognitively normal elderly persons (Holmes et al., Reference Holmes2016); whereas in our study, we did not observe an altering effect of sex on the association between anxiety and depressive symptoms and amyloid deposition. An Australian group also reported that anxiety significantly moderated the relationship between amyloid burden and decline in verbal and episodic memory among 178 subjects without dementia based on a cohort analysis (Pietrzak et al., Reference Pietrzak2014). They observed that among PiB+ participants, those with increased anxiety symptoms had a significantly greater decline as compared to those without increased anxiety symptoms (Pietrzak et al., Reference Pietrzak2014). When the study was replicated one year later with a larger sample size of 333 participants, the researchers again observed that anxiety symptoms but not depressive symptoms moderated the effect of Aß-related cognitive decline (Pietrzak et al., Reference Pietrzak2015). This is in line with our finding, as we observed a stronger association between amyloid deposition and anxiety as compared to the association between amyloid deposition and depression. Additionally, a study derived from the AD Neuroimaging Initiative (ADNI) database and involving MCI patients found that Aß+ subjects that presented with coexistent depressive symptoms had a higher risk of developing AD at a faster conversion rate than non-depressed counterparts. Moreover, the amount of deposited amyloid correlated with the rate of progression in depressed individuals (Brendel et al., Reference Brendel, Pogarell, Xiong, Delker, Bartenstein and Rominger2015). In contrast to studies that reported a relationship between neuropsychiatric symptoms and amyloid deposition, a study among patients with MCI and AD failed to detect an association between cortical amyloid deposition and depressive symptoms (Chung et al., Reference Chung2016). Our findings may be pertinent to the recently reported construct of mild behavioral impairment (MBI) which is defined as a change in behavior or personality in persons without dementia aged 50 years and older (Ismail et al., Reference Ismail2016). In our study, we observed an association between depressive and anxiety symptoms with cortical amyloid deposition. Some of the participants may have had a lifelong history of NPS. However, those that had new-onset NPS after the age of 50 can be classified as MBI; and these MBI subjects may have higher odds of having abnormal amyloid deposition in the brain. Furthermore, MBI subjects with elevated amyloid deposition may need to be closely followed in order to determine if they are at higher risk for developing incident dementia as compared to MBI subjects without cortical amyloid deposition. Previous research has shown that PiB retention may in part be mediated by PiB sulfation via estrogen sulfotransferase (Cole et al., Reference Cole2010), an enzyme involved in brain inflammation. Therefore, one possible explanation of our study findings can be an association between depressive and anxiety symptoms and brain inflammation. However, future research is needed to investigate underlying mechanisms linking amyloid deposition with depressive and anxiety symptoms. Our findings should be interpreted in the context of the strengths and limitations of the study. Strengths of our study include the large scale, population-based sample size and the categorical and continuous assessment of depressive and anxiety symptoms. The main limitation of this analysis was the low number of participants with either depressive or anxiety symptoms, even though this was expected in this population-based study sample. This limited our power to detect associations between depressive and anxiety symptoms with elevated amyloid PET SUVR. Another limitation of the study is that both BAI and BDI-II are self-reported questionnaires, thus we cannot rule out recall bias. However, both assessments are widely used for clinical research purpose. Also, as in any cross-sectional study, the direction of causality between the association of amyloid deposition and depression and anxiety is unknown. In summary, we observed an informative albeit weak association between increased BDI and BAI scores and elevated cortical amyloid deposition. This observation needs to be tested by a longitudinal cohort study.
Conflict of interest
None.
Description of authors’ roles
Formulation of the research question: Krell-Roesch, Geda. Study design: Roberts, Mielke, Vemuri, Jack, Knopman, Boeve, Petersen, Geda. Conduct of the study: Lowe, Roberts, Mielke, Vemuri, Jack, Knopman, Boeve, Petersen, Geda. Data analysis: Christianson, Kremers. Drafting of manuscript: Krell-Roesch, Geda. Critical revision of manuscript: Lowe, Neureiter, Pink, Roberts, Mielke, Vemuri, Stokin, Jack, Knopman, Boeve, Petersen.







