Article contents
Accelerated microwave-assisted synthesis and in situ X-ray scattering of tungsten-substituted vanadium dioxide (V1−xWxO2)
Published online by Cambridge University Press: 23 September 2020
Abstract
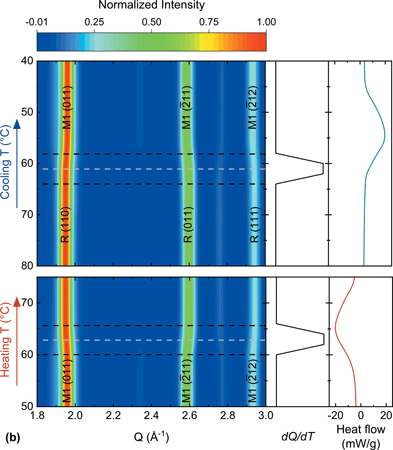
Vanadium dioxide (VO2) has been widely studied due to its metal-insulator phase transition at 68 °C, below which it is a semiconducting monoclinic phase, P21/c, and above it is a metallic tetragonal phase, P42/mnm. Substituting vanadium with transition metals allows transition temperature tunability. An accelerated microwave-assisted synthesis for VO2 and 5d tungsten-substituted VO2 presented herein decreased synthesis time by three orders of magnitude while maintaining phase purity, particle size, and transition character. Tungsten substitution amount was determined using inductively coupled plasma-optical emission spectroscopy. Differential scanning calorimetry, superconducting quantum interference device measurements, and in situ heating and cooling experiments monitored through synchrotron X-ray diffraction (XRD) confirmed the transition temperature decreased with increased tungsten substitution. Scanning electron microscopy analyzed through the line-intercept method produced an average particle size of 3–5 μm. Average structure and local structure phase purity was determined through the Rietveld analysis of synchrotron XRD and the least-squares refinement of pair distribution function data.
Information
- Type
- Invited Feature Paper
- Information
- Copyright
- Copyright © Materials Research Society 2020
Footnotes
These authors contributed equally to this work.
References
- 4
- Cited by


