Article contents
Surface and bulk characterization of Rh/ZrO2 prepared by absorption of Rh4(CO)12 clusters on ZrO2 powder
Published online by Cambridge University Press: 03 February 2012
Abstract
Rh nanoparticles supported on ZrO2 powder were prepared by adsorbing Rh4(CO)12 clusters from hexane solution under Ar atmosphere. Four samples with Rh content ranging from 0.25 Rh wt.% up to 4.10 Rh wt.% were studied by x-ray diffraction (XRD), transmission electron microscopy (TEM), x-ray photoelectron spectroscopy (XPS), scanning Auger microscopy (SAM), and time of flight secondary ion mass spectroscopy (ToF-SIMS). TEM measurements show, for all the samples, Rh particles with a size of about 50 Å. The 4.10 Rh wt.% sample also shows some agglomerates of Rh nanoparticles and only for this sample Rh metallic phase was detected by XRD. The profile analysis of the XRD lines indicates an average size of the Rh crystallites of about 60 Å. XPS studies show only a single spectral component for the Zr3d5/2 core line at 182.2 eV. Instead, at least, two components at 307.2 eV and 308.5 eV are detected for the Rh3d5/2 core line. These results suggest that Zr is present only as oxidized state, whereas nonoxidized and oxidized Rh are both observed. A nonoxidized Rh state is also suggested by the XPS valence band electron removal spectrum which exhibits a significant emission within the ZrO2 band gap assigned to Rh 4d bands. A further support to this finding arises from scanning Auger maps of OKVV and RH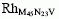 where nonoxidized Rh is observed. Finally scanning Auger maps using the OKVV and the Zr
where nonoxidized Rh is observed. Finally scanning Auger maps using the OKVV and the Zr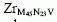 emissions show surface regions where only the Zr Auger lines are detected, whereas on pure ZrO2 powder this effect is not observed. Since it is possible to rule out from the XPS Zr3d core line spectra, the presence of metallic zirconium hydrogen spillover mechanisms is invoked to explain this result.
emissions show surface regions where only the Zr Auger lines are detected, whereas on pure ZrO2 powder this effect is not observed. Since it is possible to rule out from the XPS Zr3d core line spectra, the presence of metallic zirconium hydrogen spillover mechanisms is invoked to explain this result.
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 1997
References
- 3
- Cited by

