INTRODUCTION
Climatic warming is a primary driver of change in ecosystems worldwide (Gruner et al., Reference Gruner, Bracken, Berger, Eriksson, Gamfeldt, Matthiessen, Moorthi, Sommer and Hillebrand2017, and references therein). One of the predicted effects of the global increase of temperature is the poleward range expansion of plant and animal species, both on land and in the sea (Burrows et al., Reference Burrows, Schoeman, Buckley, Moore, Poloczanska, Brander, Brown, Bruno, Duarte, Halpern, Holding, Kappel, Kiessling, O'Connor, Pandolfi, Parmesan, Schwing, Sydeman and Richardson2011). In the world ocean, evidence of this phenomenon is accumulating especially for warm-temperate areas (Bianchi et al., Reference Bianchi, Morri, Sartoni and Wirtz1998; Vergés et al., Reference Vergés, Steinberg, Hay, Poore, Campbell, Ballesteros, Heck, Booth, Coleman, Feary, Figueira, Langlois, Marzinelli, Mizerek, Mumby, Nakamura, Roughan, van Sebille, Gupta, Smale, Tomas, Wernberg and Wilson2014; Kaimuddin et al., Reference Kaimuddin, Laë and Tito De Morais2016).
In the Mediterranean Sea, in particular, seawater warming (Shaltout & Omstedt, Reference Shaltout and Omstedt2014) has even more dramatic effects, as it offers further scope to the spread of (sub)tropical non-indigenous species (NIS hereafter) coming from the Red Sea through the Suez Canal, a man-made seaway, or from the Atlantic through the Straits of Gibraltar, a natural seaway (Bianchi & Morri, Reference Bianchi and Morri2003). The concurrent temperature increase and abundance of (sub)tropical species is leading to the so-called ‘tropicalization’ of the Mediterranean Sea, which is dramatically obvious in the south-eastern sectors of the basin. At the same time, the colder northern sectors of the basin have been said to undergo a process of ‘meridionalization’ (Boero et al., Reference Boero, Féral, Azzurro, Cardin, Riedel, Despalatović, Munda, Moschella, Zaouali, Fonda Umani, Theocharis, Wiltshire and Briand2008, and references therein), that is the arrival of warm-water natives (WWN hereafter) previously restricted to the southern sectors. Coll et al. (Reference Coll, Piroddi, Steenbeek, Kaschner, Ben Rais Lasram, Aguzzi, Ballesteros, Bianchi, Corbera, Dailianis, Danovaro, Estrada, Froglia, Galil, Gasol, Gertwagen, Gil, Guilhaumon, Kesner-Reyes, Kitsos, Koukouras, Lampadariou, Laxamana, López-Fé de la Cuadra, Lotze, Martin, Mouillot, Oro, Raicevich, Rius-Barile, Saiz-Salinas, San Vicente, Somot, Templado, Turon, Vafidis, Villanueva and Voultsiadou2010) followed Boero et al. (Reference Boero, Féral, Azzurro, Cardin, Riedel, Despalatović, Munda, Moschella, Zaouali, Fonda Umani, Theocharis, Wiltshire and Briand2008) in considering tropicalization and meridionalization as two distinct processes. Bianchi (Reference Bianchi2007) observed that the patterns of distribution change are similar for all warm-water species, whether recently introduced in or native to the Mediterranean, and are governed by the same climatic, hydrological and ecological factors. Historically, the February surface isotherms of 15 and 14°C acted as the ‘divides’ between a warmer and a colder water biota within the Mediterranean. In particular, the 15°C isotherm set the limit for the tropical species, whereas the 14°C isotherm set the limit for the native warm-water species (Bianchi et al., Reference Bianchi, Morri, Chiantore, Montefalcone, Parravicini, Rovere and Stambler2012). However, since the mid 1980s, when the Mediterranean seawater warming became evident, many ‘southerners’, either NIS or WWN, started crossing these divides to colonize even the northern reaches of the Mediterranean Sea (Bianchi, Reference Bianchi2007).
The Ligurian Sea, located in the north-western Mediterranean, is one of the coldest sectors of the basin: accordingly, its biota is nearly deprived of (sub)tropical elements (Bianchi & Morri, Reference Bianchi and Morri2000). Finding southern species, however, has become comparatively frequent in the last decades (Parravicini et al., Reference Parravicini, Mangialajo, Mousseau, Peirano, Morri, Montefalcone, Francour, Kulbicki and Bianchi2015, and references therein). All these records, however, derive from occasional observations made irregularly while diving for research not especially devoted to the occurrence of southern species: no specific monitoring programme has ever been implemented, and time data series are almost non-existent (Bianchi, Reference Bianchi2001).
Besides updating the occasional records of southern species sighted by diving in the Ligurian Sea, this paper aims at presenting the results of the first monitoring activity purposely dedicated to the inventory of southerners, either WWN or NIS. Southerners’ occurrences will be discussed in relation with temperature data, under the hypothesis that, should the difference between meridionalization and tropicalization be tenable, colonization by WWN will be more important than that by NIS.
MATERIALS AND METHODS
Study area
The area of study corresponds to the wider Gulf of Genoa, located in the northernmost part of the Ligurian Sea, from the French border to the west to the boundary with Tuscany to the east. If the ‘sub-Atlantic’ northern Adriatic, where the Mediterranean influence is reduced (Sacchi et al., Reference Sacchi, Bianchi, Morri, Occhipinti Ambrogi and Sconfietti1985), is excluded, the Ligurian Sea represents the northernmost reach for southern species in the Mediterranean Sea. Locations of this kind, where no further range expansion is possible for poleward moving species, have been called ‘range termini’ (Canning-Clode & Carlton, Reference Canning-Clode and Carlton2017).
The coasts of the Ligurian Sea are among the most urbanized and industrialized of Italy (Morri & Bianchi, Reference Morri, Bianchi, Faranda, Guglielmo and Spezie2001) but nevertheless host a number of Marine Protected Areas and Sites of Community Interest because of their importance for biodiversity conservation (Cattaneo-Vietti et al., Reference Cattaneo-Vietti, Albertelli, Aliani, Bava, Bavestrello, Benedetti Cecchi, Bianchi, Bozzo, Capello, Castellano, Cerrano, Chiantore, Corradi, Cocito, Cutroneo, Diviacco, Fabiano, Faimali, Ferrari, Gasparini, Locritani, Mangialajo, Marin, Moreno, Morri, Orsi Relini, Pane, Paoli, Petrillo, Povero, Pronzato, Relini, Santangelo, Tucci, Tunesi, Vacchi, Vassallo, Vezzulli and Wurtz2010; Parravicini et al., Reference Parravicini, Micheli, Montefalcone, Morri, Villa, Castellano, Povero and Bianchi2013). We compiled the recent records of southern species from various kinds of survey carried out by scuba diving mostly in those localities, and in particular (from west to east) in Ventimiglia, Gallinara Island, Borgio Verezzi, Bergeggi, Genoa, Portofino, Sestri Levante, Monterosso and La Spezia (Figure 1A). Species collected by sampling, with fishery, on fouling panels, or from ship hulls, and inconspicuous species found in these or other sites were not considered, as our study does not aim at a full review of all the southern species recorded from the area but just at analysing a homogeneous list of a subset of conspicuous species that had been sighted by diving.

Fig. 1. Study area. (A) the wider Gulf of Genoa in the Ligurian Sea (NW Mediterranean), with the localities where southern species have been occasionally recorded by diving in recent years. (B) Lido and Quarto, the two monitoring locales of Genoa, 2009–2015.
Purpose-done monitoring has been carried out by snorkelling in two locales of Genoa city, about 2 km from each other: Lido (44°23′21.20″N 8°58′06.50″E) and Quarto (44°23′25.69″N 8°59′22.39″E) (Figure 1B).
Field methods
The occasional records taken into account come exclusively from ecological surveys using scuba diving. Dives consisted of depth transects (Bianchi et al., Reference Bianchi, Pronzato, Cattaneo-Vietti, Benedetti-Cecchi, Morri, Pansini, Chemello, Milazzo, Fraschetti, Terlizzi, Peirano, Salvati, Benzoni, Calcinai, Cerrano and Bavestrello2004), i.e. transects deployed perpendicularly to the coastline from the water surface down to 30+ m depth. During each dive, WWN and NIS were visually identified and their depth and habitat of occurrence were noted. Only conspicuous species, easily recognizable in the field, were considered; voucher specimens were collected only in cases of doubt. Recent data (up to 2015) have been collated to update previously existing inventories (Bianchi & Morri, Reference Bianchi and Morri1993; Bianchi et al., Reference Bianchi, Montefalcone, Morri and Parravicini2011; Parravicini et al., Reference Parravicini, Mangialajo, Mousseau, Peirano, Morri, Montefalcone, Francour, Kulbicki and Bianchi2015; and references therein), and the resulting information was reorganized by 5-year periods in order to investigate potential time trends. Given the different goals of the surveys and the inhomogeneity of data available, no careful estimation of the sampling effort can be done, and interannual comparison needs to be taken with caution.
On the contrary, the finding of southern species during the monitoring activities at Genoa has been made purposely with a standardized protocol for 7 years (2009–2015 included). Species were inventoried by snorkelling in shallow (0 to about 3 m depth) rocky reefs in both Lido and Quarto, spending about 2 h per week from June to September. Species occurrences, together with other observations, were annotated on a diving slate, collection of specimens being done only when necessary to check species identity.
Temperature data
Sea surface temperature (SST) was derived from NOAA satellite data at 42°54′N and 9°24′E (freely available at www.esrl.noaa.gov/psd/cgi-bin/data/timeseries/timeseries1.pl), calibrated with the available field measurements (Bianchi & Morri, Reference Bianchi and Morri2004a). Despite calibration, these data must be considered as representative mostly of offshore conditions. In shallow coastal waters, air temperature data are often better climatic proxies than ocean temperature data (Morri & Bianchi, Reference Morri, Bianchi, Faranda, Guglielmo and Spezie2001). Thus, we also used the annual means of air temperature for the last five decades obtained from the Meteorological Observatory of Genoa.
RESULTS
Update of occasional records
Recent surveys in the wider Gulf of Genoa recorded 18 southern species, viz. 9 WWN and 9 NIS (Table 1). Among the WWN, all but Diplodus cervinus and Sparisoma cretense had already been found previously. Regarding D. cervinus, a subadult of 20 cm was observed on a shallow rocky shoal at Vesima (a locale of Genoa) in August 2008 and a juvenile at Lido in August 2011; through summer 2015, pairs or small shoals of large adults have been a common sight around the islands of Gallinara and Bergeggi. Regarding S. cretense, a single male of 22 cm was observed in June 2015 at Ventimiglia, close to the French border. In the case of Sphyraena viridensis and Thalassoma pavo, finding juveniles through the years in multiple localities has been frequent.
Table 1. New records of southern species (either NIS or WWN) by diving in the Gulf of Genoa, including the results of monitoring by snorkelling in the Genoa locales (Lido and Quarto) and further unpublished information from other Ligurian localities (Ventimiglia, Gallinara Island, Borgio Verezzi, Bergeggi, Genoa, Portofino, Sestri Levante, Monterosso, and La Spezia).
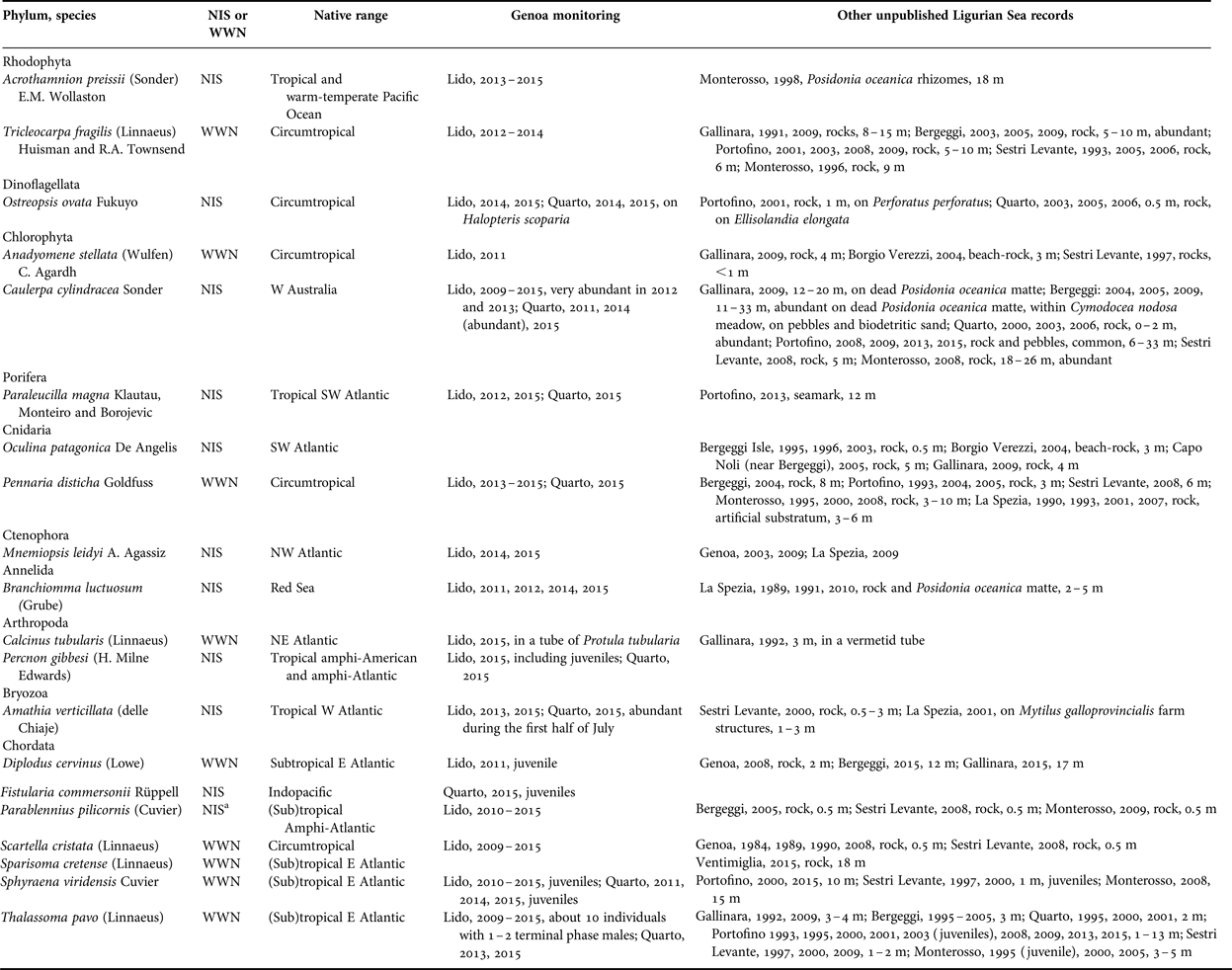
a The origin of this species is controversial (see Evans et al., Reference Evans, Barbara and Schembri2015, for a critical commentary). Here we follow Pastor & Francour (Reference Pastor and Francour2010) who consider it as non-indigenous species.
Among the NIS, four species come from the Atlantic Ocean, two from the Pacific, one from the Indian (Red Sea), and one is circumtropical (Table 1). Paraleucilla magna (Figure 3C) and Branchiomma luctuosum (Figure 3E) are new records for the Ligurian Sea. Amathia verticillata (Figure 3F) was found for the first time outside harbours in 2000.
Collating this information from recent surveys with similar occasional records by scuba diving already published in previous years, indicates that WWN tend to overnumber NIS (Figure 2). However, the number of WWN somehow fluctuated with time, with a minimum between the mid 1960s and the early 1980s, while that of NIS increased more steadily, with an apparent acceleration after the 2000s.
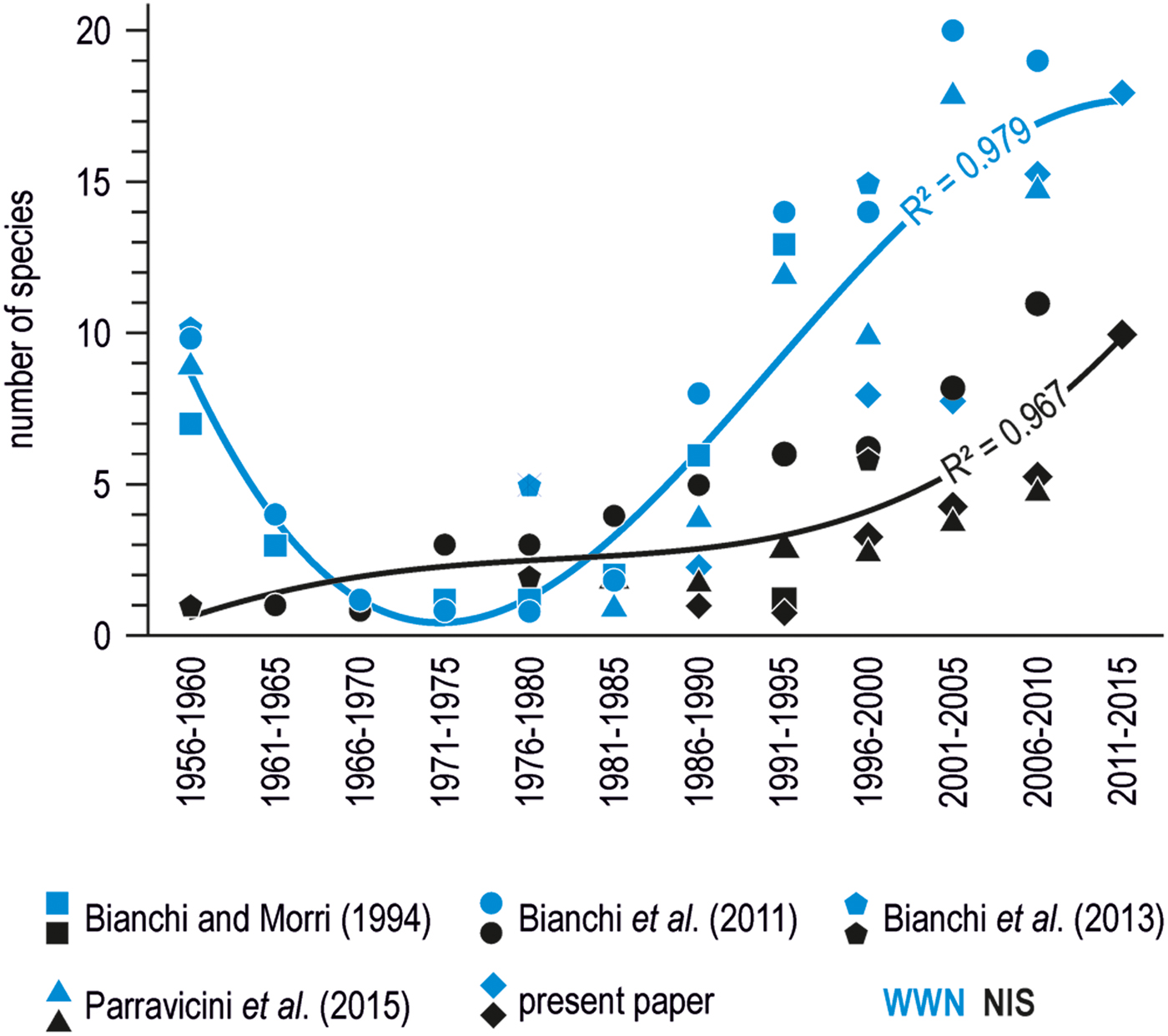
Fig. 2. Number of southern species (either WWN or NIS) recorded by diving in the wider Gulf of Genoa per 5-year period, according to various sources (Bianchi & Morri, Reference Bianchi and Morri1994; Bianchi et al., Reference Bianchi, Montefalcone, Morri and Parravicini2011, Reference Bianchi, Boudouresque, Francour, Morri, Parravicini, Templado and Zenetos2013; Parravicini et al., Reference Parravicini, Mangialajo, Mousseau, Peirano, Morri, Montefalcone, Francour, Kulbicki and Bianchi2015). The curves represent the second degree polynomial fits of mean data points for either WWN or NIS.

Fig. 3. Selected examples of southern species (either WWN or NIS) found in the Genoa monitoring locales: (A) Percnon gibbesi; (B) Sphyraena viridensis; (C) Paraleucilla magna; (D) Fistularia commersonii, juvenile; (E) Branchiomma luctuosum; (F) Amathia verticillata; (G) Pennaria disticha; (H) Scartella cristata, juvenile; (I) Parablennius pilicornis, amid Caulerpa cylindracea.
Monitoring
Monitoring by snorkelling at Genoa between 2009 and 2015 allowed inventorying 18 southern species, viz. 8 WWN and 10 NIS (Table 1, Figure 3). All the WWN but D. cervinus were already known for the Ligurian Sea. Thalassoma pavo was comparatively abundant, and terminal phase males were always observed towards the end of the summer at Lido. Also S. viridensis was a constant presence, with juveniles growing from 8 cm in June to 12+ cm in September at Quarto. In 2015 at Lido, a school of individuals about 20 cm long (Figure 3B) was characterized by a yellow tail that was reminiscent of Sphyraena flavicauda Rüppell, a Lessepsian migrant reported from Israel and Turkey but not yet from the Western Mediterranean. However, the respective position of the dorsal, pectoral and pelvic fins and the presence of a dark edge on the sides of the caudal fin gave evidence in favour of S. viridensis.
As for the NIS, five species come from the Atlantic, two from the Indian, two from the Pacific, and one is circumtropical (Table 1). Percnon gibbesi (Figure 3A) is a new record for the Gulf of Genoa and the northernmost record for the Mediterranean. The small population observed at Lido in 2015 included juveniles and females; at Quarto in 2015, we observed males fighting (Figure 4), which is possibly indicative of sexual activity, but no pregnant females. Juveniles (about 10 cm) of F. commersonii (Figure 3D) were observed for the first time in the Ligurian Sea at Quarto in 2015.

Fig. 4. A male specimen of Percnon gibbesi mutilated and wounded (arrows) during a fight with another male at Quarto, August 2015.
The number of both WWN and NIS increased with time between 2009 and 2015, but the increase was tendentially linear for the former and exponential for the latter (Figure 5).
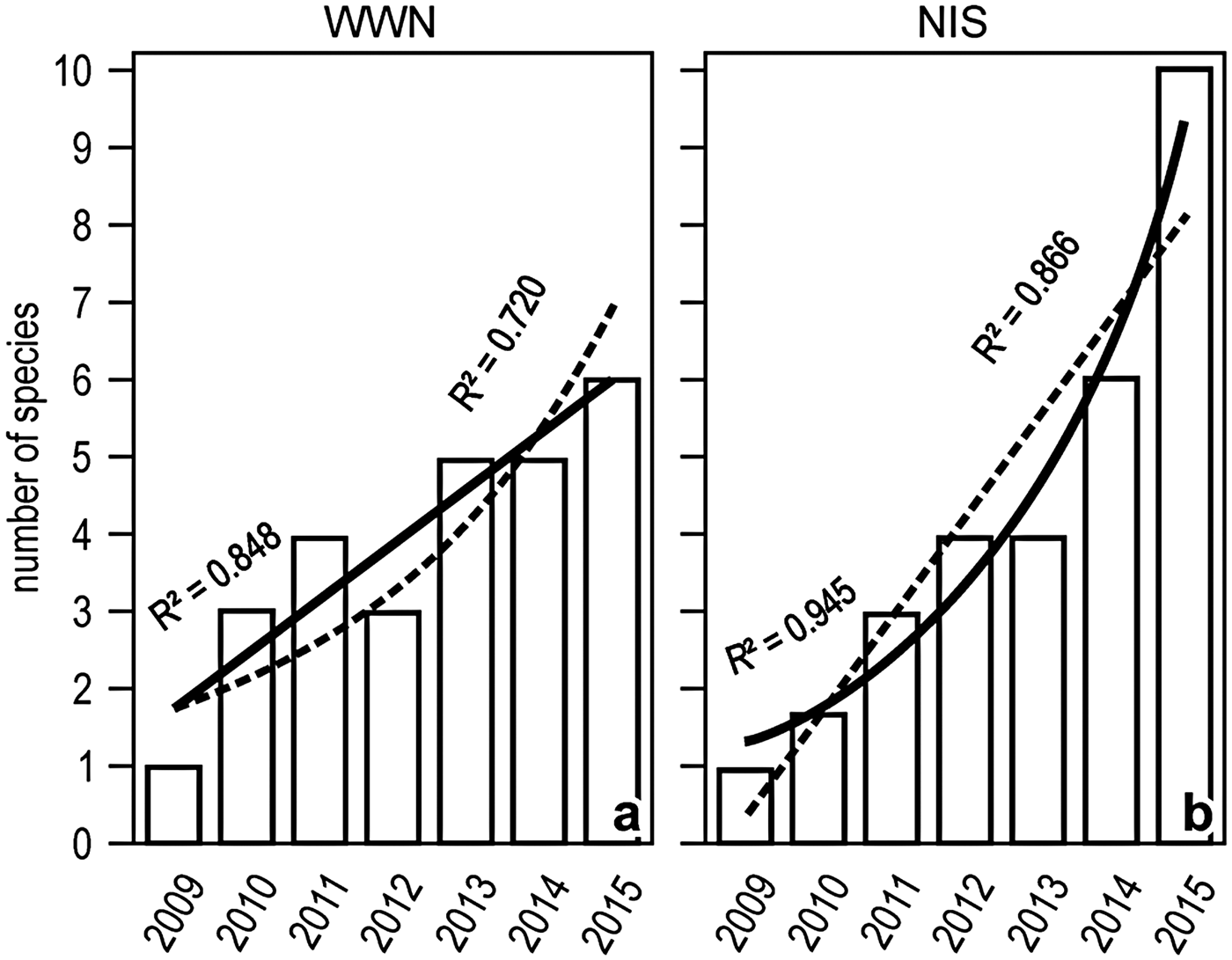
Fig. 5. Yearly trends in the number of WWN (A) or NIS (B) in the Genoa monitoring locales. Linear and exponential fits are superimposed, with solid lines indicating the best fit.
Comparison with temperature data
Both air temperature at Genoa and Ligurian Sea surface temperature raised in the last decades, average temperatures in the last years being up to half a degree higher than in the 1970s (Figure 6). However, the rise of temperature was not regular with time. Notwithstanding large year-to-year variability, four main phases can be distinguished in both air and sea surface temperature trends (Figure 6): (1) a comparatively cold phase from the late 1960s to the early 1980s; (2) a rapid warming in the second half of the 1980s; (3) a warmer, stabilized phase since the 1990s; (4) another rapid temperature rise in the last few years. At present, the fourth phase may only be guessed, and further measurements through the years to come will be necessary to confirm or contradict this hypothesis.
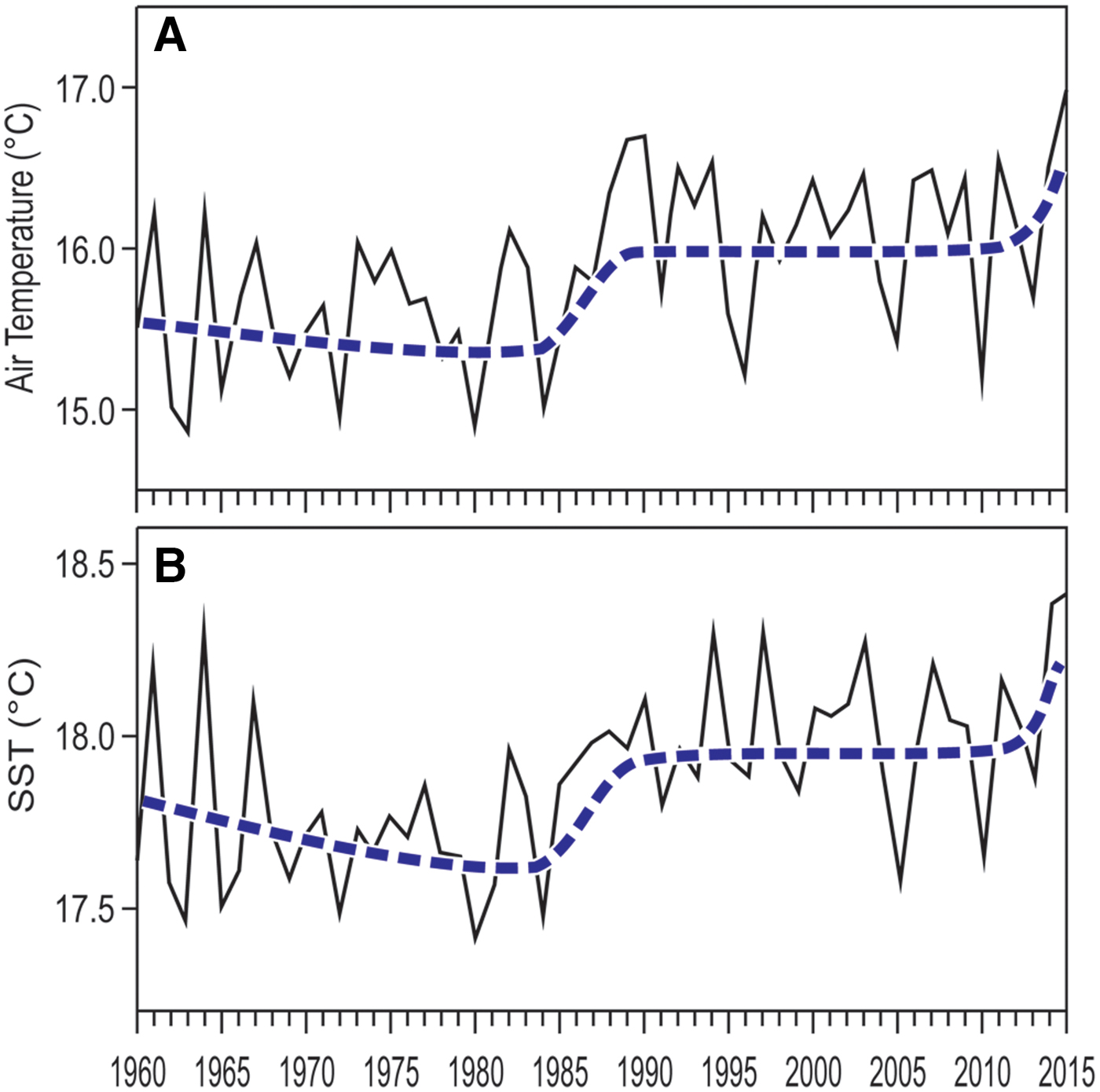
Fig. 6. Temperature yearly averages, 1960–2015. (A) air temperature, from the Meteorological Observatory of Genoa University. (B) sea surface temperature (SST), from NOAA satellite data (http:// www.esrl.noaa.gov/psd/cgi-bin/data/timeseries/timeseries1.pl). In both cases, the smoothed and broken thick line depicts the 11-year moving average.
Focussing on this last period of apparent rapid warming, and considering only the information from Genoa monitoring (more reliable than the occasional records), the total number of southern species is positively related to sea surface temperature (Figure 7A). However, the NIS-to-WWN ratio is also positively related to sea surface temperature, suggesting that warming is actually favouring NIS more than WWN (Figure 7B).
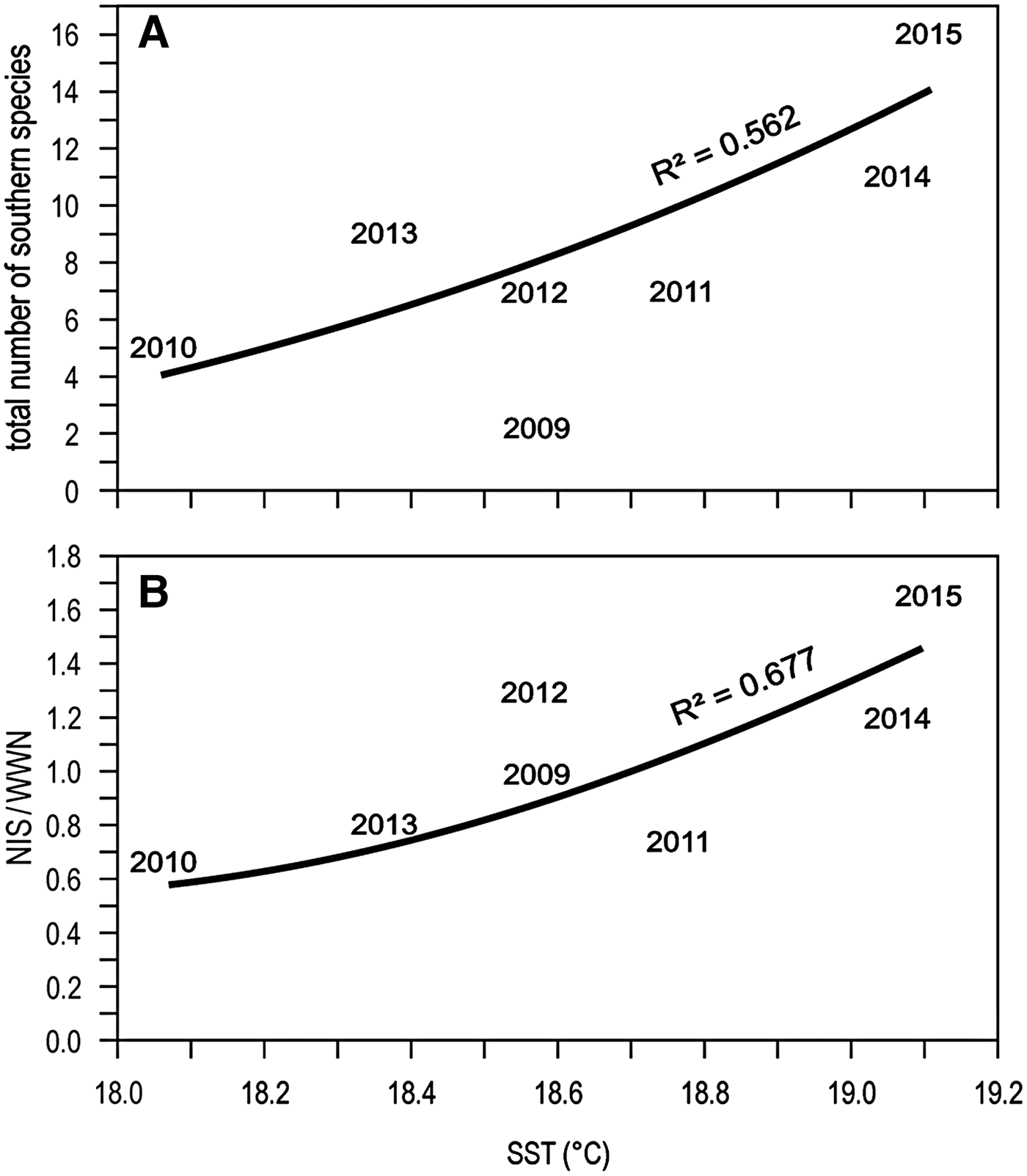
Fig. 7. Relationships between the total number of southern species (A) or the NIS-to-WWN ratio (B) found in the Genoa monitoring locales and sea surface temperature (SST). The exponential curves represent the best fit.
DISCUSSION
Collating occasional records by scuba diving from various localities of the wider Gulf of Genoa and purposely monitoring by snorkelling for 7 years (2009–2015) at two Genoa city locales (Lido and Quarto) led to a total of 20 southern species (11 NIS and 9 WWN), and allowed adding five species to the lists available in previous publications (Bianchi & Morri, Reference Bianchi and Morri1993, Reference Bianchi and Morri1994; Cattaneo-Vietti et al., Reference Cattaneo-Vietti, Albertelli, Aliani, Bava, Bavestrello, Benedetti Cecchi, Bianchi, Bozzo, Capello, Castellano, Cerrano, Chiantore, Corradi, Cocito, Cutroneo, Diviacco, Fabiano, Faimali, Ferrari, Gasparini, Locritani, Mangialajo, Marin, Moreno, Morri, Orsi Relini, Pane, Paoli, Petrillo, Povero, Pronzato, Relini, Santangelo, Tucci, Tunesi, Vacchi, Vassallo, Vezzulli and Wurtz2010; Occhipinti-Ambrogi et al., Reference Occhipinti-Ambrogi, Marchini, Cantone, Castelli, Chimenz, Cormaci, Froglia, Furnari, Gambi, Giaccone, Giangrande, Gravili, Mastrototaro, Mazziotti, Orsi-Relini and Piraino2011; Parravicini et al., Reference Parravicini, Mangialajo, Mousseau, Peirano, Morri, Montefalcone, Francour, Kulbicki and Bianchi2015; and references therein).
Two of these new records concern native species, namely the fishes Diplodus cervinus and Sparisoma cretense. Of the former, both juveniles and adults have been observed; of the latter, only one subadult. Juveniles of S. cretense have been recently seen in the French part of the Ligurian Sea (Astruch et al., Reference Astruch, Bonhomme, Goujard, Rouanet, Boudouresque, Harmelin and Harmelin-Vivien2016). Comparable northward range expansions of this species in other areas (Dulčić & Pallaoro, Reference Dulčić and Pallaoro2001; Guidetti & Boero, Reference Guidetti and Boero2001; Abecasis et al., Reference Abecasis, Bentes, Ribeiro, Machado, Oliveira, Veiga, Gonçalves and Erzini2008; Perdikaris et al., Reference Perdikaris, Konstantinidis and Paschos2012; Gambi et al., Reference Gambi, Lorenti, Patti and Zupo2016; Yapici et al., Reference Yapici, Filiz and Bilge2016) suggest a general ongoing process. Sparisoma cretense is one of the best know examples of a disjunct Atlantic-Levantine distribution (Bianchi et al., Reference Bianchi, Morri, Chiantore, Montefalcone, Parravicini, Rovere and Stambler2012). Should the present trend persist, the Atlantic Ocean and Levant Sea populations, which have been separated since the last interglacial, will merge again in the future.
The other three new records correspond to NIS: the SW Atlantic sponge Paraleucilla magna, the Red Sea polychaete Branchiomma luctuosum, and the amphi-American and amphi-Atlantic crab Percnon gibbesi. These NIS have been found on artificial substrates and/or in degraded habitats, confirming their greater susceptibility to invasion (Occhipinti-Ambrogi et al., Reference Occhipinti-Ambrogi, Marchini, Cantone, Castelli, Chimenz, Cormaci, Froglia, Furnari, Gambi, Giaccone, Giangrande, Gravili, Mastrototaro, Mazziotti, Orsi-Relini and Piraino2011). Paraleucilla magna was first recorded on an offshore seamark bearing hydrophones installed in the Marine Protected Area of Portofino to monitor dolphin activity (Brunoldi et al., Reference Brunoldi, Bozzini, Casale, Corvisiero, Grosso, Magnoli, Alessi, Bianchi, Mandich, Morri, Povero, Wurtz, Melchiorre, Viano, Cappanera, Fanciulli, Bei, Stasi and Taiuti2016). In the Tyrrhenian Sea, it similarly occurs mostly on artificial structures (Gambi et al., Reference Gambi, Lorenti, Patti and Zupo2016). Branchiomma luctuosum was first recorded in a degraded seagrass meadow and on artificial reefs in the Gulf of La Spezia in 1989 and 1991, and then was not found again until reappearing on artificial reefs at Genoa more than 20 years later. The same erratic behaviour is apparently shown in the Tyrrhenian Sea (Gambi et al., Reference Gambi, Lorenti, Patti and Zupo2016). The branchial crowns of the specimens seen in the Gulf of Genoa displayed two different colour varieties (Figure 3E): brown (more frequent at Genoa) and purple (more frequent at La Spezia). The first individuals of P. gibbesi have been observed in August 2015 amidst the blocks of artificial reefs at Genoa (Figure 3A), a preferred substrate for the larvae of this species to settle (Zenone et al., Reference Zenone, Badalamenti, Giacalone, Musco, Pipitone, Vega-Fernández and D'Anna2016).
The Indo-Pacific fish Fistularia commersonii was already known for the Ligurian Sea (Garibaldi & Relini-Orsi, Reference Garibaldi and Relini-Orsi2008; Occhipinti-Ambrogi & Galil, Reference Occhipinti-Ambrogi and Galil2008), but only as adults; juveniles were first observed in 2015 at Genoa (Figure 3D), which also constitutes the northernmost record for the species. Juveniles have also been observed for many other southern species (either WWN or NIS), and this might suggest their naturalization in the northern Gulf of Genoa. Mating and established populations there have been documented, for example, in the case of the native thermophilic fish Thalassoma pavo (Vacchi et al., Reference Vacchi, Sara, Morri, Modena, La Mesa, Guidetti and Bianchi1999, Reference Vacchi, Morri, Modena, La Mesa and Bianchi2001; Sara et al., Reference Sara, Bianchi and Morri2005).
Most of the species recorded are now widespread in the wider Gulf of Genoa, although not all reach the densities commonly observed in the Southern Mediterranean (e.g. Guidetti et al., Reference Guidetti, Bianchi, La Mesa, Modena, Morri, Sara and Vacchi2002). NIS, in particular, after the initial colonization in anthropized habitats, have recently also occupied natural habitats. The bryozoan Amathia verticillata, known for a long time as restricted to harbours (Geraci & Relini, Reference Geraci and Relini1970), has been observed off open coasts since at least 2000, in agreement with records from southern Mediterranean localities (Galil & Gevili, Reference Galil and Gevili2014; Gambi et al., Reference Gambi, Lorenti, Patti and Zupo2016). The fish Parablennius pilicornis is now common in the artificial reefs of the wider Gulf of Genoa, and its settling on natural reefs is facilitated by human impacts (Parravicini et al., Reference Parravicini, Donato, Morri, Villa and Bianchi2008, and references therein). The green alga Caulerpa cylindracea, first recorded in the Gulf of Genoa in the 1990s (Modena et al., Reference Modena, Matricardi, Vacchi and Guidetti2000, and references therein), is now common in various coastal habitats, from the sea surface to 40+ m of depth, thus contributing to the biotic homogenization of Ligurian Sea ecosystems (Montefalcone et al., Reference Montefalcone, Morri, Parravicini and Bianchi2015).
Recent experiences in the Southern Mediterranean showed that C. cylindracea provided the largest contribution to the diet of P. gibbesi (Marić et al., Reference Marić, De Troch, Occhipinti-Ambrogi and Olenin2016). Should that be true also for the Gulf of Genoa, it would raise two contrasting hypotheses: (i) the present abundance and commonness of C. cylindracea will facilitate the spread of P. gibbesi to other habitats; (ii) the establishment of P. gibbesi will control the abundance of C. cylindracea, at least at shallow depth. Further studies might elucidate these two possibilities, which perhaps might not be mutually exclusive.
There is a striking coincidence between the increase in air and sea surface temperatures and the increase in the number of records of southern species in the Gulf of Genoa. A link between seawater warming and the entry of tropical species has been demonstrated in the Eastern Mediterranean (Raitsos et al., Reference Raitsos, Beaugrand, Georgopoulos, Zenetos, Pancucci-Papadopoulou, Theocharis and Papathanassiou2010; Mavruk et al., Reference Mavruk, Bengil, Yeldan, Manasirli and Avsar2017), and the results of the present study suggest this holds true for the wider Gulf of Genoa, considering all warm-water species, either natives or NIS. Of course, it is not the temperature per se that facilitates the arrival of southern species, which may instead be favoured by changing current patterns (Gaylord & Gaines, Reference Gaylord and Gaines2000; Booth et al., Reference Booth, Figueira, Gregson, Brown and Beretta2007; Wilson et al., Reference Wilson, Fulton, McC Hogg, Joyce, Radford and Fraser2016). This is particularly true in the case of WWN (Astraldi et al., Reference Astraldi, Bianchi, Gasparini and Morri1995), whereas in the case of NIS transport on ship hulls or ballast waters and other anthropogenic vectors play a major role (Zenetos et al., Reference Zenetos, Gofas, Morri, Rosso, Violanti, García Raso, Çinar, Almogi-Labin, Ates, Azzurro, Ballesteros, Bianchi, Bilecenoglu, Gambi, Giangrande, Gravili, Hyams-Kaphzan, Karachle, Katsanevakis, Lipej, Mastrototaro, Mineur, Pancucci-Papadopoulou, Ramos-Esplá, Salas, San Martín, Sfriso, Streftaris and Verlaque2012). However, higher temperatures are obviously instrumental to the establishment success of warm-water species (Mieszkowska et al., Reference Mieszkowska, Kendall, Hawkins, Leaper, Williamson, Hardman-Mountford and Southward2006), as confirmed by the positive relation found in Genoa monitoring data.
Temperature increase in the Gulf of Genoa has not been regular: rather, several phases could be distinguished (Parravicini et al., Reference Parravicini, Mangialajo, Mousseau, Peirano, Morri, Montefalcone, Francour, Kulbicki and Bianchi2015; Gatti et al., Reference Gatti, Bianchi, Montefalcone, Venturini, Diviacco and Morri2017). Occasional records indicate a decreased richness of WWN, but not of NIS, during the comparatively cold phase of the 1970s. The rapid warming phase of the 1980–1990s caused mass mortalities in many autochthonous organisms (Cerrano et al., Reference Cerrano, Bavestrello, Bianchi, Cattaneo-Vietti, Bava, Morganti, Morri, Picco, Sara, Schiaparelli, Siccardi and Sponga2000; Lejeusne et al., Reference Lejeusne, Chevaldonné, Pergent-Martini, Boudouresque and Pérez2010), which were often replaced by NIS causing phase shift in the communities involved (Montefalcone et al., Reference Montefalcone, Morri, Peirano, Albertelli and Bianchi2007; Gatti et al., Reference Gatti, Bianchi, Parravicini, Rovere, Peirano, Montefalcone, Massa and Morri2015). Other autochthonous species found refuge at depth (Puce et al., Reference Puce, Bavestrello, Di Camillo and Boero2009; Gatti et al., Reference Gatti, Bianchi, Montefalcone, Venturini, Diviacco and Morri2017). Warm-water species have been seen to increase along a south-north gradient (Azzurro et al., Reference Azzurro, Moschella and Maynou2011) and to outcompete, where abundant, their cool-water relatives, causing depth distribution shifts (Milazzo et al., Reference Milazzo, Quattrocchi, Azzurro, Palmeri, Chemello, Di Franco, Guidetti, Sala, Sciandra, Badalamenti and García-Charton2016). It is too early to tell whether the high temperatures measured in the last 2 years are indicative of a further phase of rapid warming (Shaltout & Omstedt, Reference Shaltout and Omstedt2014). However, new events of mass mortality have been observed in the Western Mediterranean Sea in late summer 2015 (Rubio-Portillo et al., Reference Rubio-Portillo, Izquierdo-Muñoz, Gagoc, Rosselló-Morac, Antón and Ramos-Esplá2016) and a new wave of southern species established in summer 2015 in the Gulf of Genoa, their northernmost reach in the Western Mediterranean.
CONCLUSIONS
Occasional records by diving suggest that today (2015) native WWN are as numerous as NIS (nine species each) in the Gulf of Genoa. Presence-only records have been demonstrated adequate for modelling the niche of invasive species (Raybaud et al., Reference Raybaud, Beaugrand, Dewarumez and Luczak2015), but due to their inherent inhomogeneity such data should always be taken with caution. Lack of time data series (which may also validate the reliability of models) remains a major problem when investigating the biological response to climate change in the Mediterranean Sea (Bianchi, Reference Bianchi1997; Bianchi & Morri, Reference Bianchi and Morri2004b). Regular Genoa monitoring by snorkelling in the last 7 years inventoried fewer WWN than NIS (8 vs 10). In addition, and more importantly, the number of the WWN increased linearly, that of the NIS increased exponentially.
These results provide little support to the idea of meridionalization of the northern sectors of the Mediterranean Sea. In our opinion, the process of tropicalization also concerns the northern Mediterranean, where it is simply less showy: so far, tropical NIS remain few when compared to what is observed in the southern sectors (e.g. Pancucci-Papadopoulou et al., Reference Pancucci-Papadopoulou, Raitsos and Corsini-Foka2012; Evans et al., Reference Evans, Barbara and Schembri2015; Mannino et al., Reference Mannino, Parasporo, Crocetta and Balistreri2016; Turan et al., Reference Turan, Erguden and Gürlek2016). This notwithstanding, the northern Ligurian Sea is one of the areas most impacted by NIS in the Mediterranean Sea (Katsanevakis et al., Reference Katsanevakis, Tempera and Teixeira2016). Occhipinti-Ambrogi et al. (Reference Occhipinti-Ambrogi, Marchini, Cantone, Castelli, Chimenz, Cormaci, Froglia, Furnari, Gambi, Giaccone, Giangrande, Gravili, Mastrototaro, Mazziotti, Orsi-Relini and Piraino2011) listed 38 species for the Italian part of the Ligurian Sea, roughly corresponding to our wider Gulf of Genoa.
The results of Genoa monitoring seem to support the idea of Bianchi (Reference Bianchi2007) that meridionalization and tropicalization are not two distinct processes, as affirmed by Boero et al. (Reference Boero, Féral, Azzurro, Cardin, Riedel, Despalatović, Munda, Moschella, Zaouali, Fonda Umani, Theocharis, Wiltshire and Briand2008). Rather, they are an expression of the same phenomenon of poleward range extension that is occurring globally. We also raise doubts about the semantic distinction between the two terms. Both southerners (WWN and NIS) are mostly comprised of eurythermal tropical species that are able to expand their range into warm temperate seas (as the Mediterranean is) during warmer climatic phases (Briggs, Reference Briggs1974). Surely, the penetration into the Mediterranean of Red Sea species through the Suez Canal is a new phenomenon caused by the artificial cut of a new seaway between two seas that had been separated for 10 million years (Galil, Reference Galil, Golash, Galil and Cohen2006). Once in the Mediterranean, however, these Red Sea species expand their range northward in the same way as the NIS coming from the Atlantic and the WWN. The cases of Percnon gibbesi and Fistularia commersonii are paradigmatic: the former species came from the Atlantic, the latter from the Indopacific, but adding the present records to the maps published by Katsanevakis et al. (Reference Katsanevakis, Poursanidis, Yokes, Mačić, Beqiraj, Kashta, Sghaier, Zakhama-Sraieb, Benamer, Bitar, Bouzaza, Magni, Bianchi, Tsiakkiros and Zenetos2011) and Azzurro et al. (Reference Azzurro, Soto, Garofalo and Maynou2013), respectively, their distributions within the Mediterranean are today identical. If we drop the distinction NIS/WWN in favour of a tropical/warm-temperate one, the 18 species found during Genoa monitoring include 12 tropical vs six warm-temperate species (Table 1), thus reinforcing the idea that the Gulf of Genoa is getting tropicalized rather than meridionalized. To complicate the picture, the Genoa NIS also include some warm-temperate species, such as the green alga Caulerpa cylindracea and the combjelly Mnemiopsis leidyi. Another complication arises from the fact that species having previously been considered natives to the Mediterranean have recently been revealed to be NIS, such as for instance the fish Parablennius pilicornis (Pastor & Francour, Reference Pastor and Francour2010; but see also Evans et al., Reference Evans, Barbara and Schembri2015), the bryozoan Amathia verticillata (Marchini et al., Reference Marchini, Ferrario and Minchin2015), and perhaps the hydroid Pennaria disticha (González-Duarte et al., Reference González-Duarte, Megina, López-González, Galil, Goffredo and Dubinsky2016). Finally, the word ‘meridionalization’ itself may sound ambiguous: to native speakers of Latin languages, who coined it, it recalls the South (‘meridional’ meaning ‘southern’) but in English it may recall the meridians, and thus confound the native English speaking reader. Everybody gets what tropicalization and poleward spread mean, while meridionalization may be understood by Mediterranean workers only. In other regions of the NE Atlantic where poleward range extension of marine species is being studied, only the word tropicalization is used, while meridionalization is not (Kaimuddin et al., Reference Kaimuddin, Laë and Tito De Morais2016). Montero-Serra et al. (Reference Montero-Serra, Edwards and Genner2015) introduced the term ‘subtropicalization’ as an apparent equivalent of meridionalization. Poleward spread of low-latitude species is similarly observed in the southern hemisphere (Marzloff et al., Reference Marzloff, Melbourne-Thomas, Hamon, Hoshino, Jennings, Van Putten and Pecl2016): there, such species came from the North, not from the South: should we thus speak of ‘septentrionalization’ (‘septentrional’ meaning ‘northern’)? Tropicalization, meridionalization, subtropicalization and septentrionalization represent a cumbersome and pointless proliferation of terms to describe the same ecological phenomenon: the establishment of warm-water species of whatever origin in areas outside their original range due to climate warming (Canning-Clode & Carlton, Reference Canning-Clode and Carlton2017).
Should the present seawater warming continue in the future, the Mediterranean would undergo a generalized process of biotic homogenization, and the well established differentiation among its distinctive sub-basins would probably fade away (Bianchi et al., Reference Bianchi, Boudouresque, Francour, Morri, Parravicini, Templado and Zenetos2013). A different pattern of change in the southern and northern basins (i.e. tropicalization vs meridionalization) should not be expected: rather, a smooth gradient in a south-north direction will take place (Bianchi, Reference Bianchi2007). To understand and manage these changes, the organization of occasional presence-only records into structured datasets will of course remain important for quantifying species range shifts (Yalcin & Leroux, Reference Yalcin and Leroux2017) but will not be sufficient without monitoring (Lee et al., Reference Lee, Reusser, Olden, Smith, Graham, Burkett, Dukes, Piorkowski and McPhedran2008; Otero et al., Reference Otero, Cebrian, Francour, Galil and Savini2013): implementing networks of sustained monitoring activities should be a major concern for scientists and environmental managers alike.
ACKNOWLEDGEMENTS
We thank Ernesto Azzurro (Leghorn), Maria Corsini-Foka (Rhodes), Patrice Francour (Nice), Daniel Golani (Jerusalem), Leonardo Tunesi (Rome) and Marino Vacchi (Genoa) for advice about the identity of the yellow-tailed Sphyraena. Valter Capicchioni (Genoa) communicated the air temperature data from the Meteorological Observatory of Genoa. Roberta Costa (Genoa) first drew our attention to the occurrence of Fistularia commersonii and Percnon gibbesi at Quarto. Giorgio Barsotti (Genoa) helped with fieldwork and took the photo of Paraleucilla magna (Figure 3C). Jason Hall-Spencer (Plymouth) provided advice about the use of the word ‘meridionalization’ in English. Finally, thanks are due to the Director and staff of the Dinghy Snipe Club (Genoa) for logistical support during our monitoring activity at Quarto. The study of warm-water species in the Ligurian Sea fell under the frame of the project ‘The impacts of biological invasions and climate change on the biodiversity of the Mediterranean Sea’ (Italy–Israel cooperation on environment, research and development).
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.












