Introduction
Complex multifunctionality of materials is seldom achieved with monolithic single crystals. Many of the multifunctional materials found in nature are heterogeneous mixtures of widely disparate components, organized spatially from the nanometer to the micrometer scale and above, and temporally across vastly different time scales.Reference Mann1,Reference Weiner and Addadi2 A central feature of these systems is that they are all far from equilibrium during their functioning, and can yield (useful, designed) byproducts that are intricately structured and stable (though structurally frozen) approaching equilibrium.
For achieving control over spatial organization, one of the most powerful biosynthetic tools is biomineralization, a stable useful (designed) byproduct of biological activity. Collectively, biomineralization represents a class of synthetic processes in which nucleation, growth, and final morphologies of an ordering component, often inorganic, are directed, constrained, and even frustrated by an intervening “template,” typically an organized microphase of organic biomolecules. Some well-known examples are bone, cartilage, and shells.Reference Lakes3 Here, nanoscale (<10 nm) supramolecular assemblies of proteins and lipids template the organization of the inorganic phase (i.e., calcium carbonate, as lamellar aragonites at a much larger scale [<0.5 µm] in single, nanolaminated macroscopic structures). Past efforts focused on understanding the design and biological processing of these biominerals have led to a firm realization that structural hierarchy and kinetic pathways represent critical ingredients transforming otherwise simple materials such as calcium carbonate, silica, and polymers into complex ones displaying extraordinary properties, including strength, toughness, microporosity, and energy dissipation.Reference Mann1,Reference Smith, Schaffer, Viani, Thompson, Frederick, Kindt, Belcher, Stucky, Morse and Hansma4
The control of temporal organization in living systems is most frequently achieved through supramolecular self-assembly.Reference Mattia and Otto5 This endows the materials of life with the abilities to sense, respond, and adapt. Living systems generate this dynamic responsiveness by existing far from equilibrium, harnessing energy from their environment and accessing kinetic states in their free-energy landscapes, usually involving various cycles (e.g., the catalysis of adenosine triphosphate [ATP]). Structurally, they organize in modular and hierarchical fashions into a network of interacting functional modules or “interactomes.” Different from today’s synthetic material systems of interacting components, these networks reconfigure in response to external stimuli through simple recombinations and rearrangements of their constituent “modules” and subnetworks.Reference Hartwell, Hopfield, Leibler and Murray6,Reference Maslov and Sneppen7
An extraordinary example of such temporally flexible, extended modular design is the class of signal transduction machineries. Here, input from an external signal is sensitively recognized by the cell, even from a noisy and chaotic background, and transduced intracellularly to generate a cascade of chemically specific interactions between signaling proteins inside the cell. Needless to say, no single biomolecule (nor any single functional module) is responsible for signal transduction, but rather, the function arises through interactions between tens or even hundreds of modules, such as cytoplasmic protein complexes, acting in concert and with redundancy. This modular organization has proved beneficial to the cell, allowing the properties of individual modules to be robust, insensitive to environmental (or genetic) changes, while at the same time enabling environmentally sensitive dynamic changes not by de novo synthesis of new proteins, but by simply modifying the intermodule networks and connections. Thus, “decision-making” living systems are intrinsically endowed with a combination of material flexibility (or deformability), adaptability, and evolvability in their structures on the one hand and highly specific, self-amplifying, and error-correcting capacity for function on the other.
Far-from-equlibrium self-organization
From the vantage of materials science, far-from-equilibrium spatiotemporal control over self-organization during material syntheses challenges the traditional modus operandi in the synthesis of materials, which seeks to create equilibrated phases of consolidated matter—through preparation of uniform, equilibrated phases (e.g., monolithic single crystals) devoid of heterogeneities, interfaces, defects, and fluctuations—residing at the deep thermodynamic minima of their free energies and exhibiting long-term thermodynamic stability. In a sense, traditional materials science concerns the fabrication of materials that are “context-free” (having properties that are intrinsic to the material and independent of their boundaries or histories). Rather, the biological inspiration offers a rich new approach to “synthesis with design,” that is, materials whose properties depends on their “context” (how and by how far are they driven from equilibrium). Implicit in this approach is a shift in emphasis from thermodynamics to kinetic regimes in which equilibrium structures (global energy minima) based on single length scales (e.g., unit-cell dimensions in single crystals) are replaced by higher-order, nonequilibrium organizational states (Figure 1). In terms of energetics, these organized states become accessible through three generic routes.Reference Mattia and Otto5
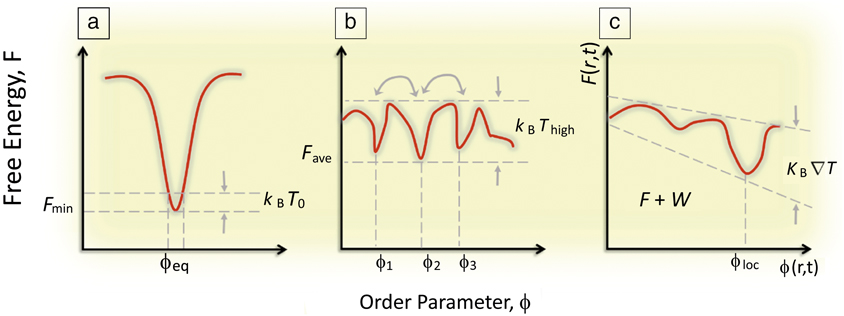
Figure 1. (a) Classical concept of a global minimization of a system’s free energy, F, which dictates the equilibrium (observed) value of some order parameter ϕ. (b) A system driven out-of-equilibrium in which F has many local minima, but no global minima and thermal energy is enough for dynamic coexisting ϕi. Note: T, temperature; k B, the Boltzmann constant. (c) A system driven far-from-equilibrium where F(r,t) varies such that the thermal energy also exhibits a gradient in r and t revealing a dynamically driven minima at ϕloc, where r is a spatial vector, t is time, and W is the work done on the system to maintain the gradient.
First, an assemblage of biomolecules comprising functional living systems exploits its compositional heterogeneity and fluctuations producing energy landscapes consisting of multiple local minima separated only by weak activation energy barriers (comparable to thermal fluctuations). These systems display temporal control of organization where the local context of the system biases the energy landscape in a feedback loop. That is, functional processes are driven by energy gradients that, in turn, help establish other energy gradients. These gradients are both temporal as well as spatial.
A particularly striking example can be found on the wing scales of butterflies,Reference Srinivasarao8 where the intricate structures lead to stunning optical effects that have fascinated scientists for centuries since the time of R. HookeReference Hooke9 and Newton.Reference Newton, Smith and Walford10 For example, the green color on the wings of the Papilio palinurus butterfly (Figure 2)Reference Vukusic and Sambles11 originates from the hierarchical microstructure of individual wing scales that are tiled on the wing. Each wing scale is about 100-µm long and composed of 4–10-µm-diameter bowls (Figure 2b–c), which in turn are lined with a multilayer stack of 11 alternating layers of air and chitin, each 75-nm thick. The distinct green color of the wings is the result of additive color mixing of yellow and blue, involving a combination of two reflections off the multilayer in the bowl; the yellow color is due to the reflection from the bottom of the bowl at normal incidence and the blue results from two 45° reflections at the edge of the bowl. The polarization of the ray reflecting from the edge is changed upon each reflection and thus the edge is visible in a reflection mode microscope under crossed polarizers (Figure 2f).
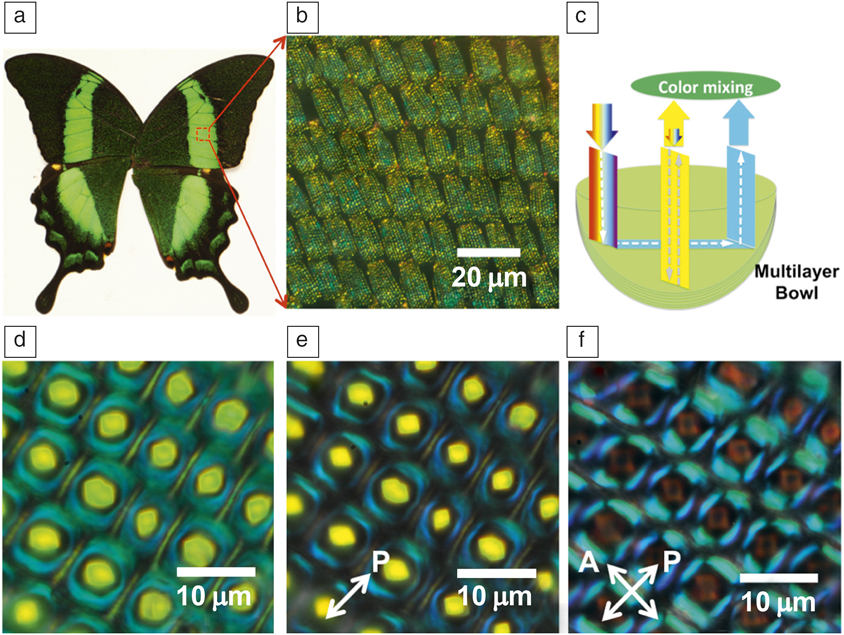
Figure 2. (a) Photograph of Papilio palinurus butterfly. (b) Tiled stack of scales of P. palinurus. (c) Schematic description of ray tracing and color mixing in an individual bowl at the green-colored area of the wing. (d–f) Optical microscope images (reflection mode) of the bowl structure on a single scale of the wing without polarizers (d), with a polarizer in the incident light path (e), and with a cross-polarized setup (f), respectively. Note: A, analyzer; P, polarizer.
Significant advances have been made in understanding the developmental underpinnings that generate wing patterns over the past couple of decades. Such studies have primarily focused on the control of early pattern elements on the wing genes that control variation in color patterns between species, and mathematical or optics-based models of how some structural colors are produced. What is not understood in our current picture of wing patterning is the developmental process that gives rise to an individual wing scale cell, a necessary primary unit for butterfly wing color, its particular shape, and the exquisitely intricate structures that pattern the wing surface and modulate or create its individual (structural) color.
Dinwiddie et. al.Reference Dinwiddie, Null, Pizzano, Chuong, Krup, Tan and Patel12 provide evidence that nonequilibrium processes or far-from-equilibrium processes lead to the formation of these intricate structures, which are eventually frozen to produce the beautiful optical effects. In the pupal stage of the scale development, each scale template produces a cytoplasm-filled extension surrounded by an active cell membrane extending out to the wing epithelium. It is also known that these begin as cylinders that become flattened during the growth phase. The authors utilized confocal microscopy to provide a detailed look at dynamic organization of F-actin (filamentous protein essential for cellular function) during the development of the scales of a butterfly. During the growth process, as the scale elongates, actin bundles become thicker and fewer in number. It should be noted that F-actin is a necessary requirement to elongate the butterfly wing scale cells, as well as for the development of the finger-like projections at the tip of the scale cells. The researchers also demonstrated that inhibiting the polymerization of actin can have disastrous effects on the development of the scale properties.
Second, spatiotemporal organization arises in many biomolecular (and nonbiomolecular) assemblies through a continuous consumption or flow of energy through the system. Called dissipative self-assembly,Reference Maiti, Fortunati, Ferrante, Scrimin and Prins13 these systems maintain ordered states as long as an external “fuel” is available. A striking example is a recent demonstration of how a simple cell-like compartment responds to sudden changes in its surroundings, such as a sudden drop in the concentration of dissolved molecules in the water that bathes the cell. This perturbation could result in a rapid flow of water into the cell through osmosis, causing it to swell, rupture, and die. To avoid this catastrophic death, even single bacterial cells have evolved mechanosensitive channel proteins, which allow them to release excess water from the cell. But how might primitive protocells handle such as assault from the environment?
Recently, Oglęcka et al.Reference Oglęcka, Rangamani, Liedberg, Kraut and Parikh14 used giant lipid vesicles to investigate how a rudimentary cell-like compartment devoid of proteins and cytosolic components responds to a sudden drop in the amount of dissolved molecules (e.g., sugar) in water. Using a synthetic, protocell-like system, consisting of only three-component mixtures of lipids, they found that these vesicular compartments respond to the osmotic assault by reorganizing the molecules at the membrane boundary and by opening a pore, which allows it to release dissolved molecules and lose excess water from the interior, thus decreasing the pressure of the encapsulated cargo. The action of this “pressure-release valve” is not all or nothing. The valve (or hole) opens for less than a second, releasing some of the internal sugar several times, producing a pulsating, breathing pattern in the size and molecular texture of the vesicle (Figure 3).15 This autonomous capability of simple vesicles to manage an external osmotic perturbation by a coordinated and cyclical sequence of physical mechanisms, which allow vesicles to sense (by lateral reorganization of membrane components and by domain formation) and regulate (by solute efflux) their local environment in a negative feedback loop, suggests a primitive form of a dissipative self-assembly in a synthetic material system (i.e., a simple microemulsion produced from just lipids, water, and sugar). From the vantage point of designing far-from-equilibrium materials, the findings suggest how chemical energy stored in concentration gradients might be controllably dissipated by cell-like compartmentalized morphologies to drive structural reorganizations, which can be exploited for new functions.
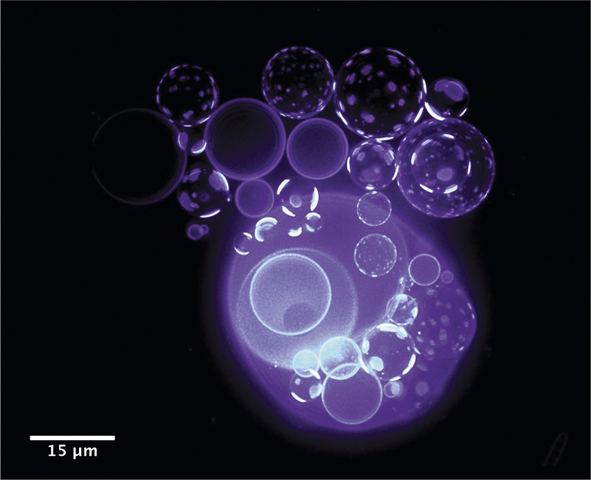
Figure 3. Coupling of osmotic activity of water (out of plane) with the membranes’ compositional degrees of freedom (in plane) results in oscillatory domain dynamics.15
Third, a novel mode of material organization involves what is now called active organization.Reference Needleman and Dogic16 Here, the “fuel” or energy flow used to access a higher-order organized state is neither external nor uniform. Instead, individual building blocks of the assemblages independently consume local chemical energy (e.g., ATP) resources (establishing internal energy gradients) achieving ordered states through widely disparate, and as yet incompletely understood, mechanisms. These systems are not perpetual, and ultimately, they rely on a net energy flow through the entire system.
An example of this third mode is the assembly and coordinated motion of individual living units such as birds, fish, bacteria, and humans, among others. (Figure 4).Reference Jansen, Cammock and Conner17 Each member is a self-contained unit responding to simple signaling among the members that confer directed organized motion or activity to the whole. While these assemblies do require flow of energy through them overall, as in the second mode (each member has to eat), the activity is entirely driven by the use of internal energy and uses gradients to even dictate roles for members on the outward facing surfaces different from those in the interior. This mode has potential far-reaching implications in understanding the early evolution of single cell organisms into multicellular organisms with specialized functions distributed among its members. While it is clear that self-assembly is occurring, it is an open question how to describe the transition from a collection of individual automata to that of a single new organism.
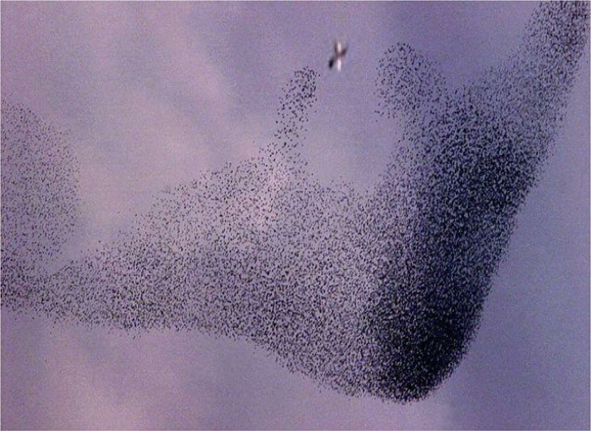
Figure 4. Example of flocking behavior of starlings in response to a threat to the group. Note the specialization of different groups of birds within the flock to respond to the predatory bird (seen in the upper center of the image).Reference Jansen, Cammock and Conner17
In this issue
All of the articles in this issue provide, in one form or another, a take on how biology produces the amazing structures with various functionalities that have garnered the attention of scientists starting with the studies of R. Hooke, who documented the intricacies of the structures found on the bodies of a variety of insects. Since that time, tremendous progress has been made in the understanding of the structures as well as their function, whether that was the intended purpose or not. In this issue, we solicited what we perceive to be at the cutting edge of the area of “bioinspired far-from-equilibrium materials.”
When one searches for materials with improved properties or entirely new functionalities or properties, the process often involves an iteration that is designed to bridge the gap between the initial starting point and the desired target. In their article, Murugan and JaegerReference Murugan and Jaeger18 believe that this can be viewed as an optimization problem with a vast search space. However, they take a different tack and point to more recent strategies exploiting knowledge about the material configuration statistics as well as highlighting the advantages of considering time-varying environments, as is often the case in biological structures.
Wilts et al.Reference Wilts, Clode, Patel and Schröder-Turk19 provide a fascinating view of the development of functional nanostructures, which are structurally frozen during development by out-of-equilibrium processes aided by actin filaments, as was documented by R. Hooke in his monograph Micrographia,Reference Hooke9 which dealt with the microstructure found on the wings of bodies of insects. The brilliant colors of many animals are a consequence of the physical interaction of light with the nanostructures that are precision-built utilizing biomaterials under ambient conditions. They note that the formation processes of nature use both elements of equilibrium self-assembly and of far-from-equilibrium and growth processes. In their article, they provide a toolbox of natural multifunctional nanostructures as well as current knowledge about the understanding of their far-from-equilibrium assembly processes.
Taking a slightly different point of view, Hess et al.Reference Hess, Katira, Riedel-Kruse and Tsitkov20 discuss the potential of molecular motors, which are motors that utilize chemical energy or light to perform work in a cyclic process, thus serving as central components for what are currently termed “active materials.” It should be noted that chemists (Sauvage, Stoddart, and Feringa) were recognized with the 2016 Nobel Prize in Chemistry for their work with the citation “for the design and synthesis of molecular machines.”21 It should be no surprise then that nature has been there before man, as is often the case. In this article, the authors highlight the machinery that nature has evolved over more than a billion years, operating close to the fundamental limits with respect to efficiency and for generation per unit mass.
In a similar vein, the article by RossReference Ross22 provides a broad overview of what she refers to as “autonomous materials from biomimicry,” where she imagines materials or autonomous systems that can sense, compute, and react to external stimuli as well as being robust. Continuing with this theme, ZocchiReference Zocchi23 provides an intriguing look at phenomena occurring at various length scales from the nanometer scale to the mesoscopic scale—these length scales have specific definitions and we prefer to leave it that way. In this case, Zocchi presents a nice tour of events from the sub-5-nm length scale to about 100 nm. He discusses processes in context, spanning molecules to neural networks, addressing basic science questions and in particular paying attention to dissipation at the atomic scale.
Finally, the article by Chatterjee and IannacchioneReference Chatterjee and Iannacchione24 addresses a well-studied system at macroscopic length scales, on the order of 20 cm with a thickness of about 7 mm. This system is the well-known, and well-studied Rayleigh–Bénard convection cell, where a thin liquid layer is subjected to a vertical temperature gradient, which results in the emergence of convection cells that are ordered. In this article, the authors explore how nonequilibrium fluctuations differ from equilibrium fluctuations.
Conclusion
Examples of synthetic materials fabricated deliberately using far-from-equilibrium synthetic strategies previously discussed are only beginning to emerge, suggesting the coming era of a new science of materials synthesis. We anticipate that understanding and exploiting nonequilibrium routes for materials synthesis will lead to new biologically motivated approaches for predictable and scalable syntheses with design, which will lead to advanced material functionalities such as self-sensing, self-healing and error-correcting, and self-replicating properties for advanced functions.

Mohan Srinivasarao is a professor at the Georgia Institute of Technology. He received his BSc degree in applied sciences from the University of Madurai, India, in 1979, and his MSc degree in applied chemistry from the University of Madras, India, in 1981. He received his MS degree in polymer science in 1985, and his PhD degree in chemistry in 1990, both from Carnegie Mellon University. He was a postdoctoral fellow at the University of Massachusetts Amherst before moving to AT&T Bell Labs. He was an assistant professor at North Carolina State University before joining Georgia Institute of Technology in 1999. He is a Fellow of the American Physical Society and the American Association for the Advancement of Science. Srinivasarao can be reached by email at mohan@mse.gatech.edu.

Germano Iannacchione is a professor of physics at the Worcester Polytechnic Institute. He received his MSc degree in physics in 1990 from The University of Akron, and his PhD degree in physics in 1993 from Kent State University. He was a Fellow at the Liquid Crystal Institute at Kent State University from 1993 to 1996, and in the Department of Chemistry at the Massachusetts Institute of Technology from 1996 to 1998. His research focuses on order-disorder phenomena in soft condensed-matter (polymers, liquid crystals, biomaterials, and colloidal composites) and far-from-equilibrium emergence. He has authored approximately 87 publications. Iannacchione can be reached by email at gsiannac@wpi.edu.

Atul Parikh is a professor and member of the faculty of the Departments of Biomedical Engineering and Materials Science & Engineering at the University of California, Davis. Since 2012, he has also served as a visiting professor in the school of Materials Science & Engineering at Nanyang Technological University, Singapore. He received his BS degree in chemistry from the University of Bombay, India, in 1987, and his PhD degree in materials science from The Pennsylvania State University in 1993. He completed postdoctoral research and was a technical staff member at Los Alamos National Laboratory from 1996 to 2001, where he explored design of biologically inspired materials and biosensors. His research interests include soft matter, membrane biophysics, and studies of the origin and chemical evolution of life. Parikh can be reached by email at anparikh@ucdavis.edu.







