Article contents
Development of magnetically active scaffolds as intrinsically-deformable bioreactors
Published online by Cambridge University Press: 27 June 2017
Abstract
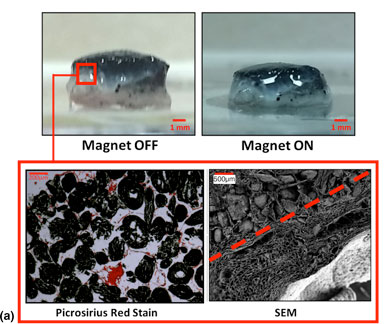
Mesenchymal stem cell behavior can be regulated through mechanical signaling, either by dynamic loading or through biomaterial properties. We developed intrinsically responsive tissue engineering scaffolds that can dynamically load cells. Porous collagen- and alginate-based scaffolds were functionalized with iron oxide to produce magnetically active scaffolds. Reversible deformations in response to magnetic stimulation of up to 50% were recorded by tuning the material properties. Cells could attach to these scaffolds and magnetically induced compressive deformation did not adversely affect viability or cause cell release. This platform should have broad application in the mechanical stimulation of cells for tissue engineering applications.
- Type
- Biomaterials for 3D Cell Biology Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 3
- Cited by




