Autism spectrum disorder and food selectivity: impact on the health status across the lifespan
Autism spectrum disorder (ASD) is a complex group of neurodevelopmental disorders such as autism, Asperger’s syndrome (AS) and pervasive developmental disorder not otherwise specified (PDD-NOS)(Reference Sharma, Gonda and Tarazi1). The most up-to-date diagnostic criteria of ASD(2) focuses on two core areas: (i) social communication impairment and (ii) restricted interests/repetitive behaviours(Reference Sharma, Gonda and Tarazi1).
Sensory processing difficulties, which encompass an increased or diminished sensitivity to the environmental sensory stimuli, also constitute another typical clinical feature(Reference Ahumada, Guzmán and Rebolledo3). Furthermore, a delay in the development of the oral motor skills is associated with ASD and may cause food refusal due to the textures that are difficult to chew or swallow(Reference Hyman, Levy and Myers4,Reference Şahan, Öztürk and Demir5) . The core sign of restrictive and repetitive behaviour, differences in sensory perception and delayed oral motor skills may contribute to feeding difficulties, resulting in food selectivity (FS)(Reference Hyman, Levy and Myers4).
Bandini et al. (2010) introduced the first and only currently available classification of FS, analysing three domains (Table 1): (i) food refusal, (ii) limited food repertoire and (iii) high frequency of single food intake(Reference Bandini, Anderson and Curtin6).
Table 1. Schematic representation of Bandini’s classification of FS(Reference Bandini, Anderson and Curtin6)

Delving into details, FS is commonly referred to as picky/fussy eating, characterised by a limited food repertoire in which the individual will experience food aversions related to specific texture, temperature, flavour, colour and odour(Reference Valenzuela-Zamora, Ramírez-Valenzuela and Ramos-Jiménez7). In addition, individuals with ASD prefer ‘predictable’ foods also known as ‘sameness’, such as foods from specific brands, not only because of the taste but mainly because they are easily recognisable due to the packaging, thus being familiar(Reference Ahumada, Guzmán and Rebolledo3,Reference Valenzuela-Zamora, Ramírez-Valenzuela and Ramos-Jiménez7) .
Evidence shows that FS is often characterised by high consumption of energy-dense ultra-processed foods poor in nutrients, causing a significant reduction in the dietary diversity and increasing the risk to develop micronutrient deficiencies(Reference Ahumada, Guzmán and Rebolledo3,Reference Valenzuela-Zamora, Ramírez-Valenzuela and Ramos-Jiménez7–Reference Luçardo, Monk and Dias9) . Nowadays scientific evidence indicates an increased risk of vitamin D deficiency among children and adolescents diagnosed with autism(Reference Wang, Ding and Wang10) as well as zinc, magnesium and calcium deficiencies(Reference Baj, Flieger and Flieger11,Reference Gallardo-Carrasco, Jiménez-Barbero and Bravo-Pastor12) .
Along with behavioural disturbances, several conditions coexist with individuals with ASD, such as gastrointestinal (GI) symptoms(Reference Valenzuela-Zamora, Ramírez-Valenzuela and Ramos-Jiménez7) which are four times more prevalent in children and adults with ASD compared with the neuro-typical population(Reference Srikantha and Mohajeri13,Reference Leader, Barrett and Ferrari14) .
In addition to the previously mentioned state, the prevalence of obesity and overweight is increasing among individuals with ASD(Reference Gilmore, Longo and Hand15): the worldwide prevalence of obesity and overweight in individuals with ASD are 21·8 % and 19·8 %, respectively(Reference Li, Xie and Lei16), and this condition could worsen their already vulnerable condition(Reference Dhaliwal, Orsso and Richard17,Reference Jones, Downing and Rinehart18) .
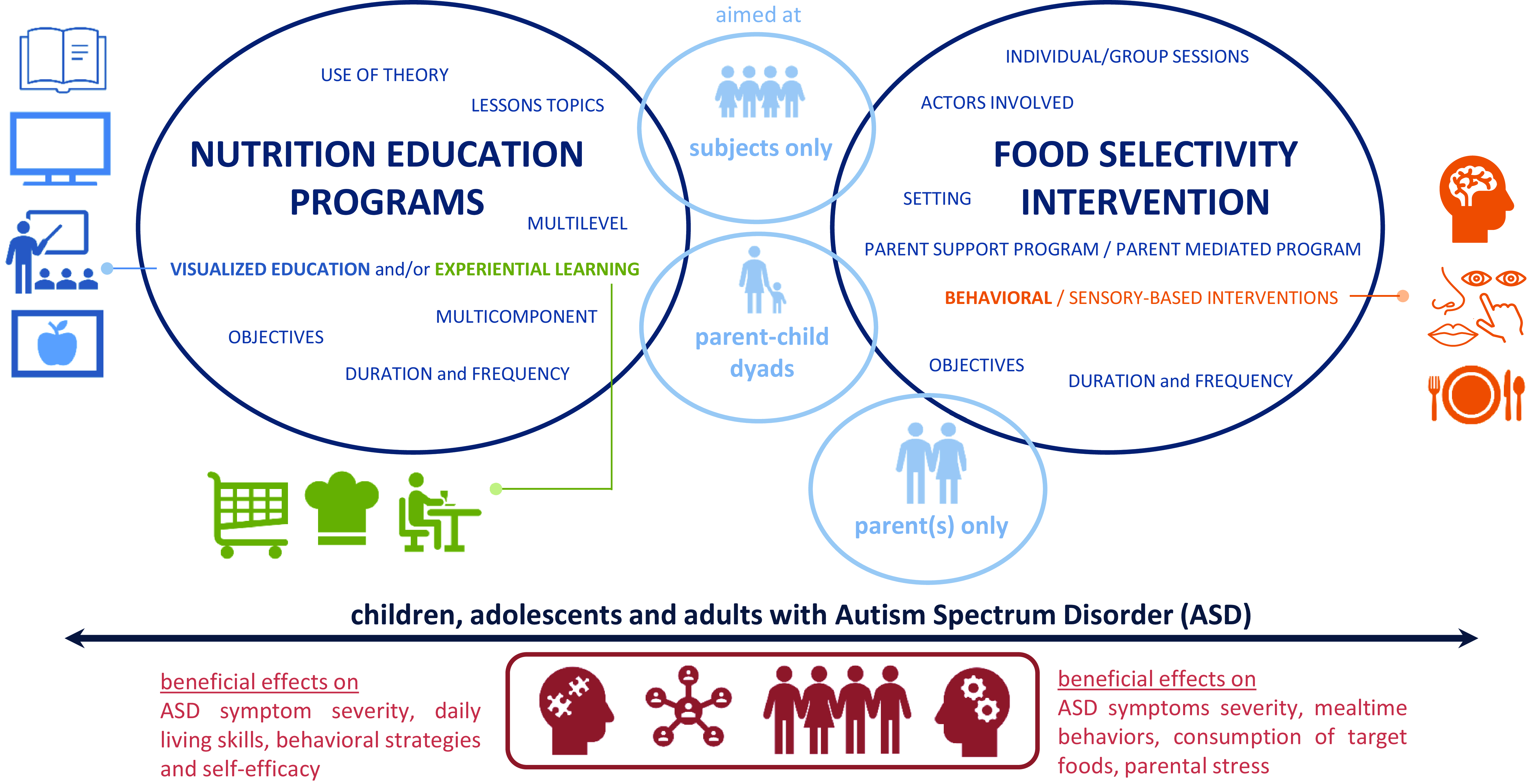
This alarming evidence mandates the creation of nutritional interventions specifically tailored for these individuals. The aim of the paper is to present an overview of the existing dietary interventions aimed at reducing FS and promoting healthy eating in individuals with ASD (Fig. 1).
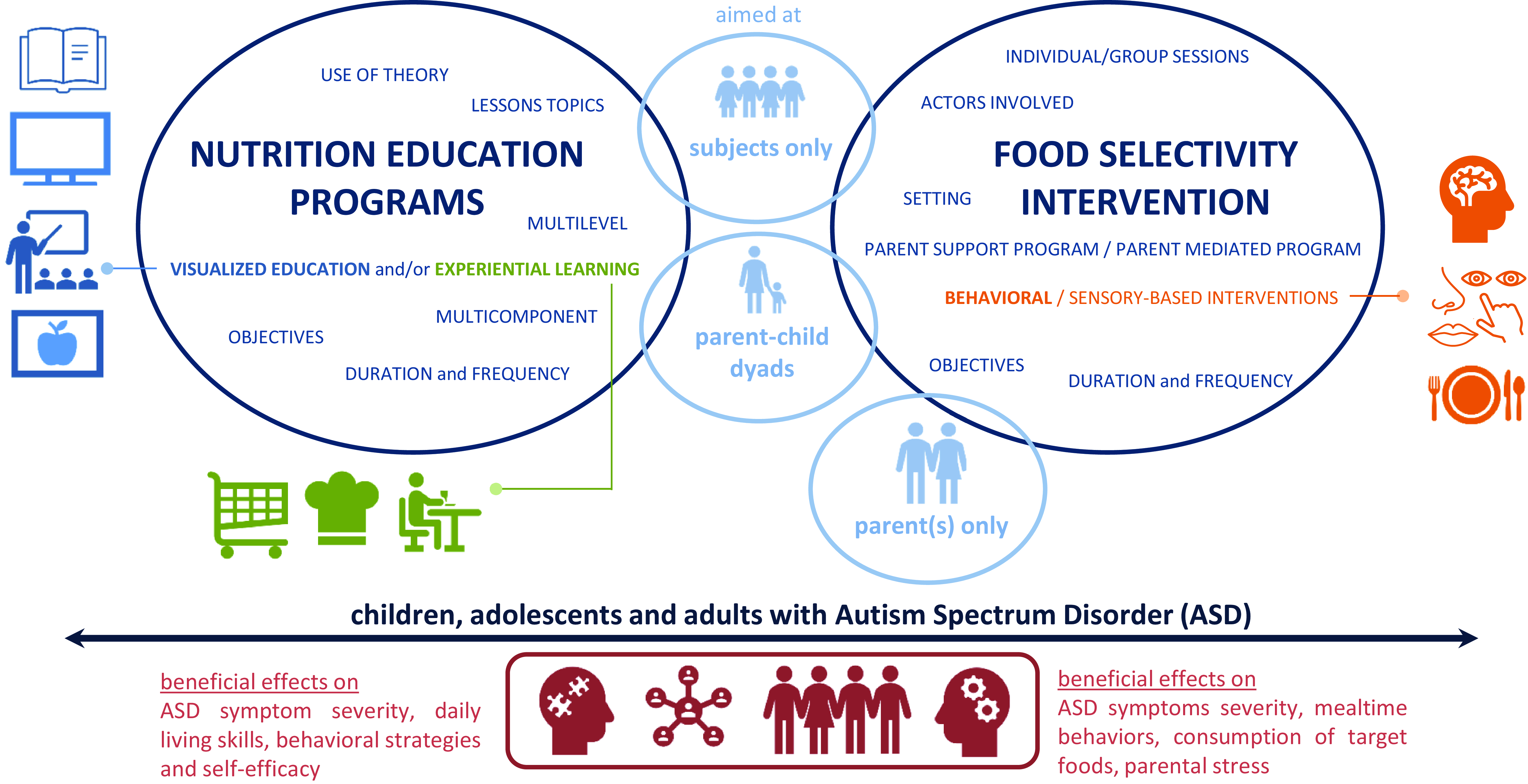
Fig. 1. Overview of the existing dietary interventions aimed at reducing FS and promoting healthy eating in individuals with ASD.
Interventions addressing food selectivity
Due to the high prevalence of FS in individuals with ASD, many specialists attempted to develop dietary intervention programmes to counteract FS(Reference Mandecka and Regulska-Ilow19). For this purpose, three main types of interventions were described in the literature: (i) behavioural interventions, (ii) sensory-based interventions and (iii) food chaining.
-
1. Behavioural interventions are currently supported by the strongest scientific evidence, and multiple studies link them to significant improvements in feeding behaviour and food consumption(Reference Marshall, Ware and Ziviani20–Reference Bloomfield, Fischer and Dove26). These evidence-based practices involve strategies such as functional assessment, positive, differential or non-contingent reinforcement, and escape extinction(Reference Cosbey and Muldoon27).
-
2. Sensory-based interventions are important since individuals with ASD are very sensitive to sensory factors, e.g. taste, texture or appearance of foods, leading to food rejection, gagging and vomiting(Reference Cosbey and Muldoon27). Moreover, systematic desensitisation is commonly used as a treatment therapy for feeding difficulties, yet it is rarely documented in the literature. It is an internally driven ‘bottom-up’ approach that involves exposure to a feared stimulus (i.e. food) while engaging in relaxation or play activities(Reference Marshall, Ware and Ziviani20,Reference Esposito, Mirizzi and Fadda28) .
-
3. Food chaining incorporates behavioural and sensory aspects of feeding, aiming at familiarising individuals with ASD with new foods that share similarities in taste, temperature or texture to the ones they already like and accept(Reference Rodrigues, Poli and Petrilli29). These food similarities are used to create ‘food chains’ or links between the foods that are considered acceptable to the child and the new ones. Based on this approach, anxiety level will be contained, enabling children to become more familiar with the new foods that will be included in their diet later on(Reference Białek-Dratwa, Szymańska and Grajek30–Reference Fishbein, Cox and Swenny32).
Other crucial considerations in managing FS include, firstly, the often-compromised oral motor skills in these individuals, which commonly contribute to the rejection of specific foods. Secondly, changes in the physical environment, such as seating arrangements, dish types and family food preferences, which could potentially impact children’s eating behaviours; this influence may manifest through modelling the consumption of fruits and vegetables, controlling snack intake and eating a wide variety of foods(Reference Hyman, Levy and Myers4,Reference Cosbey and Muldoon27,Reference Esposito, Mirizzi and Fadda28) .
It is evident that the various factors contributing to feeding difficulties require targeted interventions to be performed by different specialised professionals.
Another factor in classifying FS intervention programmes is related to the involvement of relatives/caregivers(Reference Bearss, Burrell and Stewart33,Reference Hodges, Hathaway and McMahon34) .
Bearss et al. (2015) classified the parent-training programmes for children with ASD as follows: parent support programmes (PSPs) and parent-mediated programmes (PMPs). PSPs supply parents with information and knowledge about ASD, while PMPs can be categorized as primary and complementary; in the former, caregivers facilitate the treatment from the outset, whereas in the latter, the therapist initially leads the treatment and involves the caregiver afterwards(Reference Chehade, Meyer and Beauregard31–Reference Hodges, Hathaway and McMahon34).
Parent training for FS has several advantages: it extends the intervention beyond the clinical setting, it is cost-effective since it requires less frequent clinical contact, and it has the potential to enhance the effect made during therapy(Reference Trewin, Mailloux and Schaaf35,Reference Sharp, Burrell and Jaquess36) .
In addition, greater improvements in generalisation and maintenance have been observed in cases where parents also served as therapy facilitators, and a slight tendency for improved feeding skills were observed in children whose parents received training as therapists(Reference Marshall, Ware and Ziviani20,Reference Sharp, Burrell and Jaquess36) .
The importance of parent training also resides in the fact that parents’ behaviours can contribute to the development and persistence of feeding disorders: several studies have confirmed that family behaviour generally supports the functional development of feeding in children. Nevertheless, parent–child interactions might unintentionally enforce dietary restrictions on children or contribute to limited exposure to a variety of foods(Reference Bloomfield, Fischer and Dove26,Reference Esposito, Mirizzi and Fadda28)
Moreover, parental stress can be greater in families of children with ASD, which, in itself, may lead to more behavioural changes in the child that negatively influence food selectivity(Reference Rodrigues, Poli and Petrilli29). Behavioural parent training (BPT) programmes, aimed at equipping caregivers with effective skills for managing their children’s needs, have been shown to significantly increase caregivers self-efficacy and reduce their stress levels(Reference Johnson, Brown and Hyman23,Reference Rohacek, Baxter and Sullivan24,Reference Sharp, Burrell and Jaquess36) . It is also important to note that conducting BPTs in a group setting can improve treatment availability, accessibility and engagement, and can provide a platform for caregivers to learn from one another and share common challenges(Reference Rohacek, Baxter and Sullivan24).
Materials and methods
This narrative review started with literature research in February 2023, adopting a non-systematic approach. Studies were identified from PubMed, including only English-language manuscripts published from 2014 to 2024. Study protocols, pilot studies and randomised clinical trials (RCTs) were included. Moreover, the authors reported the following keywords: nutrition education, autism spectrum disorder, parent-training, selective eating, treatment, food selectivity, program, healthy behavior, weight management, cooking, taste education, eating, feeding, caregiver, parent-delivered education, parental treatment, plan, food training, training, feeding problems.
The scientific articles were primarily selected on the basis of title/abstract screening, and then screened by full text reading. A total of 202 articles were screened by title/abstract reading, and 121 studies were excluded, while four articles were excluded by full text reading. In total, seventy-seven articles were included. The articles were excluded on the basis of the following criteria: (i) they did not address the nutritional aspect neither from a nutritional knowledge perspective nor through practical tasting sessions; (ii) they did not focus on improving the dysfunctional behaviours associated with mealtimes; (iii) they utilized other study designs.
Results
The total number of studies included in the narrative review was twenty-nine, among which fourteen were based on nutritional education programmes and fifteen on dietary interventions addressing FS. The authors summarised the results in two different tables. The first table presents the papers focusing only on nutritional education programmes (Table 2)(Reference Shurack, Garcia and Brazendale37–Reference Kral, O’Malley and Johnson54) and the second one those on dietary interventions addressing FS in individuals with ASD (Table 3)(Reference Marshall, Ware and Ziviani20–Reference Rohacek, Baxter and Sullivan24,Reference Cosbey and Muldoon27,Reference Trewin, Mailloux and Schaaf35,Reference Sharp, Burrell and Jaquess36,Reference Muldoon and Cosbey55–Reference Matheson, Drahota and Boutelle57) .
Table 2. Table summarising the education programmes targeting individuals with ASD

Legend: The programmes shown in the table are colour coded: in green are programmes aimed only at individuals with ASD, and in blue are those aimed at dyads (individual with ASD and his or her parents/caregivers). The selected cross-comparison criteria are the following: study type, sample characteristics, location, duration and frequency of the programme sessions, programme objectives, lessons topics, use of theory to develop the programme, multicomponent, multilevel, visualised education (VE) and/or experiential learning (EL). Sample characteristics: identifies the number of study participants, age and whether a weight assessment was conducted. Location: the country in which the programme was conducted. Duration and frequency: identifies the number of sessions held and the frequency with which they were conducted (e.g. weekly, biweekly…). Programme objectives: identifies the goals that the programme sets out to achieve. Lesson topics: the main thematics addressed during the sessions. Use of theory: the construction of the nutrition education programme based on a validated model for behaviour change. Multicomponent: the inclusion of several professional figures and parents to reach the educational aims. Multilevel: the inclusion of several techniques/activities. Visualised education (VE): the application of multimedia techniques to present education information in visual forms. Experiential learning (EL): the incorporation of hands-on activities.
*VE, visualised education.
†EL, experiential learning.
Table 3. Table summarising programmes targeting food selectivity in individuals with ASD

Legend: The programmes shown in the table are colour coded: in green are programmes aimed only at individuals with ASD, and in blue are those involving parents/caregivers. Area and setting: identifies the location of the study and the setting of the programme’s sessions. Duration and frequency: identifies the number of sessions held and the frequency with which they were conducted (e.g. weekly, biweekly…). Sample characteristics: identifies the number of study participants, age and whether a weight assessment was conducted. Programme objectives: identifies the goals that the programme sets out to achieve. Type of interventions: identifies the strategies implemented in the programme. Individual/group sessions: identifies whether programme sessions are group or individual. Multicomponent: identifies the actors involved during the programme sessions.
*PSP, parent support programme.
†PMP, parent-mediated programme.
Nutrition education programmes
Nutrition education is defined as ‘any set of learning experiences that are intended to facilitate the voluntary adoption of eating and other nutrition-related behaviors that are beneficial to their health and wellbeing’(Reference Murimi, Kanyi and Mupfudze58,Reference Murimi, Moyeda-Carabaza and Nguyen59) . In fact, a properly designed nutrition education programme has the potential to enhance the preference for consuming different foods and to facilitate the implementation of appropriate dietary practices(Reference Li, Huang and Yin60).
Nutrition education programmes can be classified on the basis of the presence of visual aids (visualised education, VE) and/or practical hands-on activities (experiential learning, EL)(Reference Li, Huang and Yin60–Reference Murimi, Nguyen and Moyeda-Carabaza65). Furthermore, appropriate duration (≥6 months) and frequency (weekly or biweekly) of the programmes are defined, accompanied by long-term follow-up for the maintenance of healthy habits and lasting engagement(Reference Murimi, Moyeda-Carabaza and Nguyen59,Reference Li, Huang and Yin60,Reference Angawi and Gaissi66–Reference Dewar, Lubans and Plotnikoff69) . The programme features should be multicomponent (i.e. involving several professionals), multilevel (i.e. involving several techniques/activities) and tailored to suit the age range of the participants(Reference Li, Huang and Yin60). Moreover, the programme should be guided by the application of behavioural change strategies (i.e. social cognitive theory or theory of planned behaviour or transtheoretical model of behaviour change)(Reference Murimi, Moyeda-Carabaza and Nguyen59,Reference Li, Huang and Yin60) . These reported characteristics were used to evaluate the nutrition education programmes included in this narrative review.
Considering the study analysed (Table 2), the majority of the programmes were tested as pilot studies (twelve out of fourteen)(Reference Shurack, Garcia and Brazendale37–Reference Buro, Gray and Kirby45,Reference Thorsteinsdottir, Njardvik and Bjarnason47,Reference Ketcheson and Pitchford50,Reference Manzanarez, Garcia and Iverson53) . In most of the cases, nutrition education programmes were applied in the USA (twelve out of fourteen)(Reference Shurack, Garcia and Brazendale37–Reference Garcia, Shurack, Leahy, Brazendale, Lee and Lawrence39,Reference Goldschmidt and Song41–Reference Buro, Gray and Kirby45,Reference Ketcheson and Pitchford50–Reference Kral, O’Malley and Johnson54) , which aligned with the high prevalence of ASD in the American population(Reference Zablotsky, Black and Blumberg68).
In terms of age range, interventions focusing solely on individuals with ASD targeted adolescents (15–19 years old)(Reference Shurack, Garcia and Brazendale37,Reference Garcia, Shurack, Leahy, Brazendale, Lee and Lawrence39) and/or young adults (20–31 years old)(Reference Garcia, Cathey and Shurack38,Reference Veneruso, Varallo and Franceschini40,Reference Goldschmidt and Song41) . On the other hand, interventions targeting both those with ASD and their respective families, predominantly enrolled children (5–12 years old)(Reference Kuschner, Morton and Maddox43,Reference Burrell, Sharp and Criado44,Reference Thorsteinsdottir, Njardvik and Bjarnason47,Reference Kral, O’Malley and Johnson54) . Only nine out of fourteen programmes assessed the weight of the study participants(Reference Shurack, Garcia and Brazendale37,Reference Gray, Pang and Agazzi42,Reference Burrell, Sharp and Criado44,Reference Buro, Gray and Kirby45,Reference Thorsteinsdottir, Njardvik and Bjarnason47,Reference Ketcheson and Pitchford50,Reference Espinoza, Chen and Orozco51,Reference Manzanarez, Garcia and Iverson53,Reference Kral, O’Malley and Johnson54) .
In terms of duration, the programmes consisted of 4–25 weeks of intervention provided weekly once or twice; each session lasted from 25 to 120 min.
The main objective of all nutrition education programmes, whether stated implicitly or explicitly, was to prevent the adverse health effects caused by malnutrition condition. Delving into the evaluations of the covered topics, they included (i) fundamentals of nutrition, such as instructions on balanced nutrition (i.e. caloric reduction, macro- and micronutrient, portion versus serving size, food groups and beverages, USDA MyPlate), (ii) healthy grocery shopping and (iii) proper label reading. Moreover, specific nutritional aspects about the most frequently experienced feeding difficulties by individuals with ASD were also covered, including sensory properties of food and tasting sessions, meal planning (i.e. visual mealtime routines and schedules), food environment restructuring and behaviour management strategies (i.e. cognitive behaviour techniques). Classes (in person or online) were frequently combined with hands-on workshops (i.e. cooking demonstrations, healthy foods and snack tasting sessions) and some homework to promote the integration of the knowledge gained through the practices in their daily habits at home, and to ensure its long-term maintenance. The knowledge transition promotion was highlighted by the fact that twelve out of fourteen nutrition education programmes aimed at individuals with ASD involved visualised education and experiential learning activities(Reference Shurack, Garcia and Brazendale37–Reference Veneruso, Varallo and Franceschini40,Reference Gray, Pang and Agazzi42,Reference Kuschner, Morton and Maddox43,Reference Buro, Gray and Kirby45,Reference Thorsteinsdottir, Njardvik and Bjarnason47,Reference Ketcheson and Pitchford50,Reference Espinoza, Chen and Orozco51,Reference Manzanarez, Garcia and Iverson53,Reference Kral, O’Malley and Johnson54) .
In terms of the use of theory for behavioural change, only eight out of fourteen programmes reported clearly the use of at least one theory that underlined the construction of the nutrition education program(Reference Garcia, Cathey and Shurack38,Reference Garcia, Shurack, Leahy, Brazendale, Lee and Lawrence39,Reference Goldschmidt and Song41,Reference Gray, Pang and Agazzi42,Reference Burrell, Sharp and Criado44,Reference Ketcheson and Pitchford50,Reference Espinoza, Chen and Orozco51,Reference Manzanarez, Garcia and Iverson53) .
Noteworthy was the use of a multidisciplinary approach, ensuring the involvement of multiple professionals (multicomponent), and the combination of multiple activities (multilevel). Indeed, ten out of fourteen programmes combined the two aspects(Reference Garcia, Cathey and Shurack38,Reference Garcia, Shurack, Leahy, Brazendale, Lee and Lawrence39,Reference Gray, Pang and Agazzi42–Reference Burrell, Sharp and Criado44,Reference Thorsteinsdottir, Njardvik and Bjarnason47,Reference Ketcheson and Pitchford50,Reference Espinoza, Chen and Orozco51,Reference Manzanarez, Garcia and Iverson53,Reference Kral, O’Malley and Johnson54) .
In terms of results, almost half of the programmes focused mainly on the evaluation of the feasibility and acceptability of the programme(Reference Shurack, Garcia and Brazendale37–Reference Garcia, Shurack, Leahy, Brazendale, Lee and Lawrence39,Reference Kuschner, Morton and Maddox43–Reference Buro, Gray and Kirby46) , while three others focused on the description of the programme(Reference Goldschmidt and Song41,Reference Gray, Pang and Agazzi42,Reference Ketcheson and Pitchford50) , without analysing the outcomes. In terms of domain behaviour, in the culinary programme developed by Veneruso et al. the severity of ASD symptoms and the daily living skills significantly improved(Reference Veneruso, Varallo and Franceschini40). They were assessed respectively using Childhood Autism Rating Scale, Second Edition (CARS-2) and Vineland Adaptive Behavior Scale II (VABS II) Daily Living Skills Scale(Reference Veneruso, Varallo and Franceschini40).
In the programme developed by Buro et al.(Reference Buro, Gray and Kirby45), an improvement in individuals’ behavioural strategies and self-efficacy was detected using two scales from validated SCT-based surveys(Reference Sam, Cox and Savage70). In terms of dietary intake, the programme developed by Buro and colleagues was effective in reducing the consumption of added sugar(Reference Buro, Gray and Kirby45); this was measured using the Block Kids 2004 food frequency questionnaire(Reference Buro, Gray and Kirby45). However, no post-intervention improvements were detected in the consumption of fruits and vegetables(Reference Thorsteinsdottir, Njardvik and Bjarnason47). The taste education programme of Thorsteinsdottir and colleagues confirmed that there were no statistically significant results concerning the acceptance of fruits, despite the increased odds of accepting vegetables, nuts and seeds(Reference Thorsteinsdottir, Njardvik and Bjarnason47,Reference Thorsteinsdottir, Njardvik and Bjarnason48) . However, in this study, children’s acceptance was assessed through parent-reported intake of selected food items(Reference Thorsteinsdottir, Njardvik and Bjarnason47,Reference Thorsteinsdottir, Njardvik and Bjarnason48) . Moreover, the programme showed a positive effect when compared with a waiting group, on all measures of Meals in Our Household Questionnaire. These effects remain stable through the 6 months follow-up period(Reference Thorsteinsdottir, Njardvik and Bjarnason47,Reference Thorsteinsdottir, Njardvik and Bjarnason48) .
The mobile health intervention provided by Kral et al.(Reference Kral, O’Malley and Johnson54) did not yield significant differences in terms of changes in the consumption of the targeted foods or beverages between the two groups. Only children who consumed few fruits and vegetables at baseline and who were highly engaged with the technology increased their intake post-intervention(Reference Kral, O’Malley and Johnson54).
Furthermore, the comprehensive behavioural family-based lifestyle intervention (CBFLI) programme conducted by Espinoza and colleagues showed a significant decrease in BMI Z-scores in children with ASD as in NT children without specific adaptations for the ASD population(Reference Espinoza, Deavenport-Saman and Solomon52). However, the generalisability of this result is limited by the specific study sample characteristics: low-income, Hispanic/Latino, Spanish-speaking families(Reference Espinoza, Deavenport-Saman and Solomon52).
In detail, the specific characteristics of the nutrition education programmes that were customised for individuals with ASDs are reported in Table 4.
Table 4. Schematic representation of the nutrition education programmes’ specific characteristics aimed at an appropriate declination in favour of individuals with ASDs, combined with their relative rationale

Programmes to counteract food selectivity
Analysing the programmes addressing FS (Table 3), it was observed that most of them were presented in RCT pilot studies (six out of fifteen)(Reference Johnson, Foldes and DeMand21,Reference Johnson, Brown and Hyman23,Reference Rohacek, Baxter and Sullivan24,Reference Sharp, Burrell and Jaquess36,Reference Muldoon and Cosbey55,Reference Sharp, Burrell and Berry56) , followed by RCTs (four out of fifteen)(Reference Marshall, Ware and Ziviani20,Reference Sharp, Burrell and Berry56) . All studies were conducted in the USA, with the exception of Marshal J. et al. (2015), which was conducted in Australia(Reference Marshall, Ware and Ziviani20). The sample size of the studies was small: no study surpasses the participant count of forty-four participants.
Since all the analysed programmes were addressing children (maximum age 13 years), most of them (thirteen out of fifteen)(Reference Marshall, Ware and Ziviani20,Reference Johnson, Foldes and DeMand21,Reference Johnson, Brown and Hyman23,Reference Rohacek, Baxter and Sullivan24,Reference Cosbey and Muldoon27,Reference Trewin, Mailloux and Schaaf35,Reference Sharp, Burrell and Jaquess36,Reference Muldoon and Cosbey55–Reference Matheson, Drahota and Boutelle57) involved caregivers and were divided into PMPs (ten out of thirteen)(Reference Marshall, Ware and Ziviani20,Reference Johnson, Foldes and DeMand21,Reference Johnson, Brown and Hyman23,Reference Rohacek, Baxter and Sullivan24,Reference Cosbey and Muldoon27,Reference Sharp, Burrell and Jaquess36,Reference Muldoon and Cosbey55–Reference Matheson, Drahota and Boutelle57) and PSPs (three out of thirteen)(Reference Rohacek, Baxter and Sullivan24,Reference Trewin, Mailloux and Schaaf35,Reference Sharp, Burrell and Berry56) . Parental involvement was absent in only two programmes(Reference Peterson, Piazza and Volkert22). Regarding the intervention setting of PMPs, six studies out of ten(Reference Johnson, Foldes and DeMand21,Reference Johnson, Brown and Hyman23,Reference Rohacek, Baxter and Sullivan24,Reference Cosbey and Muldoon27,Reference Sharp, Burrell and Jaquess36,Reference Matheson, Drahota and Boutelle57) were carried out in home setting, three out of ten(Reference Marshall, Ware and Ziviani20,Reference Muldoon and Cosbey55) in clinical setting, and one out of ten(Reference Sharp, Burrell and Berry56) at home and in clinical settings.
Regarding the type of intervention of PMPs, the majority of studies considered were behavioural, two out of ten(Reference Cosbey and Muldoon27,Reference Muldoon and Cosbey55) employed different strategies (behavioural, sensory-based strategies and communication support) and just one(Reference Sharp, Burrell and Berry56) was sensory-based only.
As for PSPs, two out of three(Reference Rohacek, Baxter and Sullivan24,Reference Sharp, Burrell and Berry56) were psychoeducational and supportive programmes, while one out of three(Reference Trewin, Mailloux and Schaaf35) aimed for parental education about the typical sensory integration difficulties in individuals with ASD. Concerning the two programmes that did not support parental involvement(Reference Peterson, Piazza and Volkert22), one was behavioural, whereas the other was sensory-based.
The duration and frequency of intervention varied across programmes, with the number of sessions ranging from a minimum of 6 to a maximum of 36 weeks. The session frequencies ranged from less than once a week to weekly, biweekly, three times a week and an intensive option of ten sessions in one week. Among the programmes, four out of ten(Reference Rohacek, Baxter and Sullivan24,Reference Sharp, Burrell and Jaquess36,Reference Sharp, Burrell and Berry56,Reference Matheson, Drahota and Boutelle57) PMPs and two out of three(Reference Rohacek, Baxter and Sullivan24,Reference Sharp, Burrell and Berry56) PSPs supply group sessions; while programmes that do not involve parents provide individual sessions.
The professional figures involved in the programmes were mainly psychologists, speech and language therapists, and certified therapists in applied behaviour analysis (ABA). Only two out of fifteen programmes(Reference Johnson, Brown and Hyman23,Reference Sharp, Burrell and Berry56) had the presence of dietitians. It is worth noting that anthropometric assessment was conducted in seven out of fifteen(Reference Marshall, Ware and Ziviani20,Reference Johnson, Foldes and DeMand21,Reference Sharp, Burrell and Jaquess36,Reference Muldoon and Cosbey55,Reference Matheson, Drahota and Boutelle57) programmes.
In terms of programme objectives, the fundamentals were: (i) reducing children’s challenging mealtime behaviours (12/15 programmes)(Reference Marshall, Ware and Ziviani20–Reference Rohacek, Baxter and Sullivan24,Reference Cosbey and Muldoon27,Reference Sharp, Burrell and Jaquess36,Reference Muldoon and Cosbey55,Reference Matheson, Drahota and Boutelle57) , (ii) expanding dietary variety (9/15 programmes)(Reference Marshall, Ware and Ziviani20,Reference Peterson, Piazza and Volkert22,Reference Cosbey and Muldoon27,Reference Sharp, Burrell and Jaquess36,Reference Muldoon and Cosbey55,Reference Matheson, Drahota and Boutelle57) and (iii) reducing caregivers’ stress (7/15 programmes)(Reference Johnson, Foldes and DeMand21,Reference Johnson, Brown and Hyman23,Reference Rohacek, Baxter and Sullivan24,Reference Sharp, Burrell and Jaquess36,Reference Sharp, Burrell and Berry56) .
In terms of results, studies that compared behavioural interventions with other types showed greater effectiveness of behavioural ones. In Rohacek A. et al. (2023)(Reference Rohacek, Baxter and Sullivan24), a blinded evaluator used the Clinical Global Impression-Improvement (CGI-I), a clinician-rated scale that tracks changes in global functioning and response to treatment, to compare the behavioural PMP and the PSP. CGI-I reported a statistically significant improvement in the former compared with the latter(Reference Rohacek, Baxter and Sullivan24). In addition, the rates of treatment acceptability and satisfaction were higher in the behavioural PMP and during the follow-up period, and the decrease in depressive symptoms was maintained in the behavioural PMP, whereas no such maintenance was observed in the group assigned to the PSP(Reference Rohacek, Baxter and Sullivan24). In the study by Peterson K. M. et al. (2016), children who were assigned to the behavioural intervention showed an increase in their consumption of target foods, whereas this was not observed in the group that received the sensory-based intervention(Reference Peterson, Piazza and Volkert22). However, treatment generalisation was observed in two of the three children who initially participated in the sensory intervention and were subsequently assigned to the behavioural intervention(Reference Peterson, Piazza and Volkert22). Therefore, the implementation of the behavioural intervention after the sensory intervention resulted in a potential treatment generalisation effect(Reference Peterson, Piazza and Volkert22). In the study of Sharp W. G. et al. (2019), a higher rate of improvement was observed in the behavioural PMP compared with the PSP(Reference Sharp, Burrell and Berry56). This improvement was measured using the CGI-I scale by a blinded independent evaluator(Reference Sharp, Burrell and Berry56). Meanwhile, children participating in the behavioural PMP showed significantly lower scores on the Brief Autism Mealtime Behavior Inventory (BAMBI) at both week 12 and week 16(Reference Sharp, Burrell and Berry56). The group assigned to the behavioural PMP showed an increase in the grams consumed during the meal observations at both times, and a decrease was observed in the group assigned to the PSP at each timepoint(Reference Sharp, Burrell and Berry56). At 20-week follow-up, the CGI-I score remained unchanged from week 16 levels for twelve participants of the behavioural PMP (80 %)(Reference Sharp, Burrell and Berry56). Finally, in Marshall J. et al. (2015) study favourable outcomes were achieved regardless of intervention type (behavioural or sensory-based), but although the differences were not statistically significant, it is important to note that from a clinical point of view participants in the behavioural PMP demonstrated greater improvement in dietary variety compared with the sensory-based PMP(Reference Marshall, Ware and Ziviani20).
Conclusion
Various nutritional interventions are documented in the literature aimed at individuals with ASD, including those aimed at reducing FS in individuals with ASD and those aimed at enhancing their dietary quality and food choices.
Regarding the former, no study has analysed the long-term impact. They mainly focused on assessing feasibility in improving symptoms related to social and behavioural areas, as well as dietary habits. However, programmes that aim to improve dietary quality may have limited effectiveness if the individuals they target show marked FS, so the strength of such programmes lies in implementing strategies that take this into account as well.
Considering programmes aimed at reducing FS, there is currently no gold-standard approach. However, behavioural strategies are associated with significant improvements in eating behaviour. One limitation of these programmes is that, in their effort to expand the eating repertoire of individuals with ASD, healthy foods are not consistently introduced at the outset of treatment sessions. Consequently, the path to improvement may be prolonged, necessitating the later implementation of a programme specifically targeting the enhancement of food quality.
In conclusion, this narrative review provides the reader with a better understanding of the nutrition interventions implemented to date for individuals with ASD, divided into nutrition education programmes and those aimed at reducing FS. It highlights the need to test the effectiveness of the former since this aspect is missing in the literature and provides a starting point for the implementation of further studies to define the role of sensory-based interventions, communication support and psychoeducational approaches in reducing FS.
Acknowledgements
The authors acknowledge the contribution of Dana El Masri for the English revision. Dr El Masri is affiliated with the Laboratory of Dietetics and Clinical Nutrition, Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy.
Financial support
This research was funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1·3 – Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU. Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title ‘ON Foods – Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods’.
Competing interests
The authors declare no conflicts of interest.
Authorship
Conceptualisation: Maria Vittoria Conti, Sara Santero, Chiara Breda; methodology: Maria Vittoria Conti, Sara Santero, Chiara Breda; writing – original draft preparation: Sara Santero, Chiara Breda; writing –review and editing: Maria Vittoria Conti, Sara Santero, Chiara Breda; visualisation: Hellas Cena; supervision: Hellas Cena. All authors have read and agreed to the published version of the manuscript.