Article contents
Cytochrome b as a more promising marker for analysing the distribution vector for Metagonimus suifunensis (Trematoda: Heterophyidae)
Published online by Cambridge University Press: 15 February 2021
Abstract
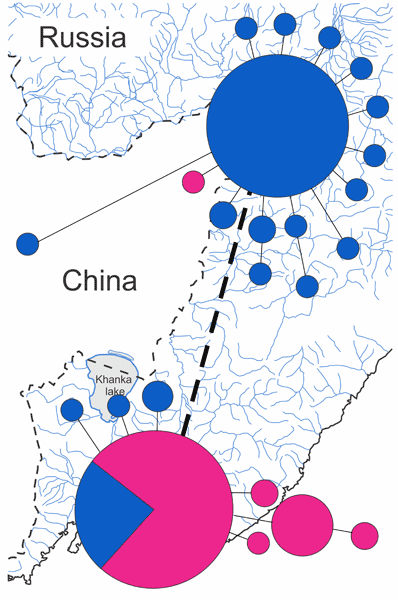
In this study of Metagonimus suifunensis (M. suifunensis) in the Russian Southern Far East, the variability of the full-length sequences of the cytochrome b (cytb) mtDNA gene was assessed for the first time. In addition, the cox1 mtDNA gene sequences were also obtained for this species from new localities. In total, 87 and 81 sequences of the cytb and cox1 genes, respectively, were used in the current study. The cytb gene proved more promising and revealed two haplogroups that are associated with the spatial distribution of the species: geographical isolation caused the fixation of differences between northern and southern populations. In addition, the results obtained for the cytb gene opened up new perspectives in the analysis of sequences of the cox1 gene, which was not sufficiently effective as a sole marker. Based on data for both mitochondrial genes, molecular processes influencing the formation of the modern population were analysed for M. suifunensis. The new data confirmed the previously expressed opinion that this species colonized the study territory from north to south and will form the basis for determining possible ways of its further expansion, which is important for predicting the emergence of new foci of metagonimosis.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 2
- Cited by





