Introduction
The Philippines is an endemic area of a myriad of neglected tropical diseases (NTDs), six of which: lymphatic filariasis, schistosomiasis, soil transmitted diseases, foodborne trematodiases, rabies and leprosy are of public health importance (Leonardo et al., Reference Leonardo, Hernandez, Magturo, Palasi, Rubite, De Cadiz and Fontanilla2020). Schistosomiasis and fascioliasis are among the notable parasitic infection shared by human and bubaline reservoir hosts. In most cases, bovines play a significant role in transmitting parasitic diseases as reservoir hosts that release thousands of parasite eggs daily in the environment, which then develop into larvae or other infective stages (Gordon, Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and Mcmanus2012; Aragaw and Tilahun, Reference Aragaw and Tilahun2019).
There are four municipalities bordering Lake Mainit, most of which have rice fields adjacent to the lake. The lake scape communities surrounding Lake Mainit have been reported endemic for schistosomiasis as early as 1947 and have hampered the lake's tourism and economy in general (Cassion et al., Reference Cassion, Pingal, Maniago and Medina2013). The rice fields strategically located adjacent to Lake Mainit were suitable nidus of active parasite transmission via bubaline reservoir hosts because farming is still mostly unmechanized (Jumawan et al., Reference Jumawan, Balamad and Estaño2020; Jumawan and Estaño, Reference Jumawan and Estaño2021). The lake-rice field interface is often extensively flooded during rainy months, which could promote the spread of zoonotic diseases through bovine fecal matter and snails serving as hosts to several parasitic species (Jumawan et al., Reference Jumawan, Estaño, Siega, Maghinay, Santillan and Jumawan2016; Aragaw and Tilahun, Reference Aragaw and Tilahun2019). Initial surveys have documented the link between snails and bovines in spreading the disease in ricefields (Jumawan and Estaño, Reference Jumawan and Estaño2021) and other bovine-associated parasitic diseases (Jumawan et al., Reference Jumawan, Balamad and Estaño2020). The occupational risk of farmers and lakeshore residents to schistosomiasis includes exposure to water bodies (irrigated canals, rice paddies, swamps and residential areas) where snails and bovines thrive (Jumawan et al., Reference Jumawan, Estaño, Siega, Maghinay, Santillan and Jumawan2016).
The Philippines’ prevention and control of schistosomiasis mainly focused on chemotherapy for human hosts (Leonardo et al., Reference Leonardo, Hernandez, Magturo, Palasi, Rubite, De Cadiz and Fontanilla2020). Nonetheless, reports of the critical role of water buffaloes as primary reservoir hosts in spreading the disease have been reported (Gray et al., Reference Gray, Copland and Copeman2008; McManus et al., Reference McManus, Gray, Ross, Williams, He and Li2011; Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and Mcmanus2012). The zoonotic nature of the disease calls for a multidisciplinary, multisectoral approach that should engage communities and their leaders, medical professionals, veterinarians, ecologists, malacologists, environmentalists and educators (Tenorio et al., Reference Tenorio, Manalo and Molina2021). An integrated approach to control the disease should include operational components such as adequate water supply and sanitation, environmental management, snail control, health education, chemotherapy (Praziquantel) and vaccination (Jumawan and Estaño, Reference Jumawan and Estaño2021).
Schistosomiasis and other zoonotic diseases in bubaline reservoir hosts remain largely unknown in the rest of the endemic foci (Tenorio et al., Reference Tenorio, Manalo and Molina2021). Additional surveys are needed to provide other vital information to raise awareness and proper management of the transmission of pathogenic parasites recovered in bovine feces in the rice fields of these areas. This study reported the updated and consistent prevalence of S. japonicum, F. gigantica and other zoonotic parasites in bovine reservoir hosts in the Lake Mainit ecosystem.
Materials and methods
Study area
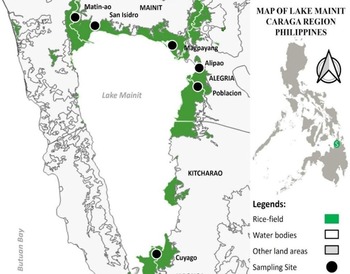
Fecal samples were collected from the rice fields of six lakeside barangays near Lake Mainit, namely the barangay Matin-ao, San Isidro, Alipao, Poblacion Alegria, Magpayang and Cuyago (Fig. 1), from August to November 2021. These shoreline barangays were chosen based on schistosomiasis cases reported from stool data and previous studies in the area (Abao-Paylangco et al., Reference Abao-Paylangco, Balamad, Paylangco, Japitana and Jumawan2019; Jumawan et al., Reference Jumawan, Balamad and Estaño2020). Additional sampling stations were also explored aside from the sampling points surveyed by Jumawan and Estaño (Reference Jumawan and Estaño2021). A geographical position satellite, model GARMIN GPS 72, was used to take the geographical locations of all sampling sites where fecal samples were collected. The map was constructed using QGIS v.3.22.1 software.

Figure 1. Map of ricefield stations in Lake Mainit, Philippines.
Collection of fecal samples from bovines
Consent from Local Government Units (LGUs), ricefield and bovine owners was obtained before fecal collection. Collection of feces was done by scooping 3–5 g of freshly fallen bovine feces (at least 24 h since defecation) using a fecal scooper and storing them in sterile containers with 2 mL of 10% formaldehyde for preservation (Jumawan et al., Reference Jumawan, Balamad and Estaño2020; Jumawan and Estaño, Reference Jumawan and Estaño2021). Scooping of samples was obtained from the upper surface of feces to avoid ground contamination. The sex and species origin of the bovine fecal sample source was not determined. Fecal samples were collected from the actual bovines exposed to grazing, foraging and farming activities in the selected ricefield stations with the aid of animal owners to ensure the feces were obtained from all bovines in each sampling site.
Formalin-ethyl-acetate sedimentation (FEA-sd) technique
The stool parasitological examination technique adopted by Jumawan and Estaño (Reference Jumawan and Estaño2021) was used in this study. This procedure utilized the novel copro-parasitological method described by Xu et al. (Reference Xu, Gordon, Hu, Mcmanus, Chen, Gray, Ju, Zeng, Gobert, Ge, Lan, Xie, Jiang, Ross, Acosta, Olveda and Feng2012) for detecting parasite eggs in bovine fecal samples, the FEA-SD., with a few modifications. A modified McMaster Egg Counting Chamber was used to read the entire volume of the sample (Jumawan and Estaño, Reference Jumawan and Estaño2021).
Statistical analysis
The parasite infection prevalence in bovines was determined based on parasite eggs/cysts in fecal samples. Egg counts in 5 g of feces were noted. The collected bovine fecal samples among stations were tested for their significant association with parasite infection prevalence using Chi-square independent test. The bovine contamination index (BCI) was determined following Gordon et al. (Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and Mcmanus2012), Tenorio and Molina (Reference Tenorio and Molina2020) and Jumawan and Estaño (Reference Jumawan and Estaño2021). Statistical computations were performed using Quantitative Parasitology (QP) version 3.0. and SPSS v. 20.0 software.
Results
Bovine fecal parasites from rice fields
Laboratory analysis recovered seven major parasites: Schistoma japonicum, Fasciola gigantica, Ascaris sp., Strongyloides sp., Balantidium coli, coccidian oocyst and hookworm species from bovine fecal samples. All collected fecal samples (N = 124) were positive for parasite infection (Table 1). The chi-square independent test revealed a significant (P = 0.001) difference in infection among recovered parasites, with F. gigantica (100%) and hookworms (53.08%) having the highest infection prevalence rates (Table 1). The rice fields of Alipao and Cuyago had the most recovered parasite species; however, fecal samples varied in the parasitic load (Table 2). The liver fluke F. gigantica had the highest egg counts among other parasites recorded from bovine fecal samples (Table 2). The present survey reveals fecal samples from the ricefields of Cuyago harbour the highest number of S. japonicum eggs with a prevalence of 44%, followed by Alipao (18.3%) and Poblacion, Alegria (9.09%), respectively.
Table 1. Prevalence rate of bovine fecal parasites from rice fields adjacent to Lake Mainit, Philippines

Table 2. Eggs/Cyst per count of bovine fecal parasites from rice fields adjacent to Lake Mainit, Philippines

Multiple parasite infection
This study recorded ten combinations of multiple infections of parasites in various rice fields adjacent to Lake Mainit (Table 3). Co-infection of F. gigantica and hookworms was the most prevalent across sampling sites. Fecal samples from the rice fields of Cuyago have the highest infection (84.2% prevalence rate). A combination of four parasite species in one fecal sample from Cuyago was documented: F. gigantica, hookworm, coccidian oocyst and Strongyloides sp. Multiple infections with three to two parasite species in various sampling sites were also noted (Table 3).
Table 3. Prevalence of multiple parasite infections in bovines from ricefields adjacent to Lake Mainit, Philippines

Bovine contamination index (BCI) for Schistosoma and Fasciola
Calculations of the BCI showed that, on average, infected bovines in key rice fields of Lake Mainit could excrete an average of 104, 750 S. japonicum eggs as deposited in the environment each day (Table 4). Bovine schistosome infection can be considered ‘light infection’ for Cuyago (2.28 MPEG), Alipao (1.1) and Poblacion, Alegria (0.8). The present survey recorded a higher BCI of approximately 104, 750 Schistosoma eggs daily. However, bovines across all rice field stations were heavily infected with fascioliasis with BCI of 162, 700 Fasciola eggs per day (Table 5). Co-infection of F. gigantica and S. japonicum eggs in fecal samples was low (11–12%; Table 3).
Table 4. Bovine contamination index (BCI) for Schistosoma in ricefields of Lake Mainit calculated using the arithmetic MEPG of the FEA-s.d. data

* Calculated using 25 kg as the daily fecal output for bovines (Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and Mcmanus2012; Tenorio and Molina, Reference Tenorio and Molina2020).
Table 5. Bovine contamination index (BCI) for Fasciola in ricefields of Lake Mainit calculated using the arithmetic MEPG of the FEA-s.d. data

* Calculated using 25 kg as the daily fecal output for bovines (Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and Mcmanus2012; Tenorio and Molina, Reference Tenorio and Molina2020).
Discussion
The ricefield is a crucial habitat for disease transmission when infected snails are present, and farmers utilize these fields unprotected (Jumawan and Estaño, Reference Jumawan and Estaño2021). The high prevalence of infection of F. gigantica, a plant-borne trematode, in the feces of bovines from rice fields is consistent with the previous report of Jumawan et al. (Reference Jumawan, Balamad and Estaño2020). Fascioliasis infection occurs when a definitive host (humans or cattle) accidentally ingests the parasite by eating raw watercress or other contaminated freshwater plants and the presence of such intermediate snail hosts (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009; Chang and Flores, Reference Chang and Flores2015; Portugaliza et al., Reference Portugaliza, Balaso, Descallar and Lañada2019).
Ascaris suum is a nematode commonly harboured in pigs and cross-infected with bovines (Taylor et al., Reference Taylor, Spagnoli, Calcutt and Kim2016). Acute lung inflammation, stomach distension and discomfort, and intestinal blockage are among the symptoms of Ascaris infections in humans. Both A. lumbricoides and A. suum infection result in abdominal distension, pain and intestinal obstruction (Bokhari, Reference Bokhari2021). In the Philippines, ascariasis is associated with strongyloidiasis in other mammalian animals infection. Strongyloides stercoralis is the pathologic agent of strongyloidiasis in humans (Baloria et al., Reference Baloria, Gamalinda, Rosal and Estaño2022). In the present survey, eggs of Strongyloides sp. were recovered from bovine fecal samples from barangay Alipao. Strongyloides spp. is a common intestinal nematode of mammalian hosts that parasitizes the small intestine and can cause diarrhoea and malnutrition, especially in young animals (Jumawan et al., Reference Jumawan, Balamad and Estaño2020).
Hookworm infection from bovine feces was also initially reported in 2020 (Jumawan et al., Reference Jumawan, Balamad and Estaño2020). This parasite inhabiting mammals’ alimentary system results in anaemia caused by the loss of iron and protein in the stomach (Maharana et al., Reference Maharana, Kumarm, Sudhakar, Behera and Patbandha2015). Their transmission and infection in humans and domestic animals are well-documented, making them a significant neglected tropical disease-causing agent affecting both primates and ruminants (Baloria et al., Reference Baloria, Gamalinda, Rosal and Estaño2022).
Balantidium coli was recovered from bubaline fecal samples in four barangays: Magpayang, Cuyago, Poblacion Alegria and Alipao. This protozoan is a common intestinal parasite of pigs and a causal agent of balantidiasis in humans, which could be attributed to backyard pig farming in these areas. Human infection is usually an uncommon occurrence caused by cyst contamination in food and water. These issues are more frequent among malnourished people, those who work with pigs, cattle and other animals, and those who work in unsanitary conditions (Kumar et al., Reference Kumar, Rajkumari, Mandal and Parija2016). Coccidia is a common intestinal parasite of pigs. Infection in livestock results in weight loss and diarrhoea and affects animal production (Tumusiime et al., Reference Tumusiime, Penrith, Githigia and Ocaido2020; Gong et al., Reference Gong, Zhao, Wang, Zhang, Liu, Huang and Zhu2021). This parasite can be a causal agent of coccidiosis, potentially infecting humans (Knight et al., Reference Knight, Mcclellan, Dufour and Hendrickson2018).
Incidences of multiple infections, such as F. gigantica, hookworm, coccidia and Strongyloides sp., in the feces of bovines, were previously reported (Jumawan et al., Reference Jumawan, Balamad and Estaño2020). Bovine fecal samples in the area recovered with Schistosoma eggs in Barangay Cuyago and Alipao (Jumawan and Estaño, Reference Jumawan and Estaño2021). Other parasites were also consistently recovered, particularly Strongyloides sp., Ascaris sp., coccidian oocysts and eggs of hookworm helminths. The current study updates recorded new combinations of multiple infections of intestinal parasites and observed higher prevalence rates of infection. The coccidian oocyst, a common avian parasite (Sood et al., Reference Sood, Singh, Kaur, Kumar and Singh2017), is consistently recovered in fecal samples collected in the ricefields of barangay Alipao, an ecotone interface of wild animals, including migratory birds, bovines and other livestock animals such as ducts, pigs and other ruminants. Emergence and cross-infection of zoonotic parasites in this habitat may take place.
The prevailing infection of Schistosoma in bovine fecal samples in the rice fields of Cuyago and Alipao shows a persistent zoonotic transmission in the area (Jumawan and Estaño (Reference Jumawan and Estaño2021). Oncomelania snails in the ricefields of Alipao harboured schistosome cercaria. In Cuyago, infected snails were found distantly from the ricefields utilized by bovines for bathing and foraging, suggesting that the ricefield is not the only nidus for schistosomiasis emergence (Jumawan and Estaño, Reference Jumawan and Estaño2021).
The earliest case of schistosomiasis in Lake Mainit was reported in 1947 by Pesigan (Reference Pesigan1947), and the occurrence has been persistently documented from random surveys of human stool samples ever since. The topographic features of Lake Mainit are suitable endemic foci where critical elements for continuous transmission are maintained (Jumawan and Estaño, Reference Jumawan and Estaño2021). The disease is considered a prevailing endemic public health concern that is endemic to Caraga and 11 other regions in the Philippines (Olveda et al., Reference Olveda, Yuesheng, Olveda, Lam, Mcmanus, Chau, Harn, Williams, Gray and Ross2014; Leonardo et al., Reference Leonardo, Chigusa, Kikuchi, Kato-Hayashi, Kawazu, Angeles, Fontanilla, Tabios, Moendeg, Goto, Fornillos, Tamayo and Chua2016). The ricefield is a crucial habitat for human schistosomiasis transmission when infected snails are present, and farmers utilize these unprotected fields. Potential high-risk exposure of humans to Schistosoma may still be possible even if bovines are absent in rice paddies and other wet areas. Infection can still occur with or without the bovine reservoir host if Oncomelania harbouring Schistosoma is present.
The survey recorded a higher BCI of approximately 104, 750 Schistosoma eggs daily compared to the previous study, with ~ 40, 000 S. japonicum eggs in the environment (Jumawan and Estaño, Reference Jumawan and Estaño2021). The increased number of BCI per individual bovines supports the claim of the previous result that the timing of the Schistosoma life cycles and egg release in the stool of bovines may have a seasonal variation. The parasite's life cycle may still prevail since Schistosoma may utilize other mammalian hosts, such as rodents, dogs, pigs and other nearby ruminants. This factor is considered an alarming eyeshot of uninterrupted transmission of Schistosomiasis in endemic foci. The extensive surveys in other wet areas, as recorded in Cuyago, bovines had the highest infection rate, proving that rice fields may be one of many sources of infection for bovines. However, areas such as those for animal grazing and resting may be potential venues for bovine schistosomiasis (Jumawan and Estaño, Reference Jumawan and Estaño2021). The current survey updated rice fields with infected bovines, particularly Poblacion Alegria. These results demonstrate that bovine zoonosis could be widespread that may serve as a source of parasites capable of infecting humans.
Fascioliasis in the Philippines has been documented most typically through bovines (Gray et al., Reference Gray, Copland and Copeman2008; Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009; Portugaliza et al., Reference Portugaliza, Balaso, Descallar and Lañada2019) but rarely in humans (Gray et al., Reference Gray, Copland and Copeman2008) where they occur due to the consumption of raw water vegetables infested with Fasciola. Culturally rooted eating behaviours and sanitation practices in endemic areas are important risk factors for acquiring and perpetuating foodborne trematodiasis, as in the case of fascioliasis (Tenorio and Molina, Reference Tenorio and Molina2021). While there are two Fasciola species in the Philippines, our current study reports the presence of F. gigantica (130–145 μm × 70–90 μm). Reports on human fascioliasis in the country are scarce and are primarily random research undertaken by undergraduate and graduate students (Leonardo et al., Reference Leonardo, Hernandez, Magturo, Palasi, Rubite, De Cadiz and Fontanilla2020). Bovine monitoring surveys by line agencies of Agusan del Norte and Surigao del Norte do not include the occurrence of schistosomiasis and fascioliasis. Some sections of Mindanao practice building bovine enclosures away from rice fields and storing and drying bovine feces before using them as fertilizer, significantly reducing schistosomiasis cases. They could also be adapted for controlling fascioliasis (Gray et al., Reference Gray, Copland and Copeman2008).
Most recovered parasitic helminths identified in the present study are classified as NTDs causing agents. The high infection of bovine fascioliasis exemplifies that topographic feature favours the zoonosis of parasitic helminth in the ricefield of the lake ecosystem as in the case of Lake Mainit. The lake-ricefield interface may facilitate the synergistic infection of other parasites, such as hookworms, Strongyloides sp., coccidian oocysts and Ascaris sp., harbouring in bovines and must be given attention for control measures of the transmission to animals and humans. Molecular-based analysis, such as environmental DNA studies, may provide additional data for detecting schistosomiasis and other bovine-mediated diseases.
Conclusion
The study provided updates on the infection of bubaline reservoir hosts in rice fields adjacent to Lake Mainit by surveying eggs and cysts of parasites from bovine feces. The significant incidence of multiple infections in fecal samples confirms the critical role of bovines as a reservoir host for schistosomiasis and other diseases in the rice fields adjacent Lake Mainit. The current study suggests conducting more research and molecular-based analysis to ensure the sensitivity and efficacy of the bubaline parasitic detection and to explore the potential zoonotic capacity of the recovered parasites. The newly auxiliary positive sites illustrate the prevailing zoonosis transmission in the lake ecosystem and call for urgent health-related interventions such as agricultural practices and environmental modification, bovine vaccination and deworming, and other integrated approaches to control and eradicate zoonotic disease transmission by bubaline reservoir hosts.
Data availability
Not applicable.
Acknowledgements
The authors are indebted to the various LGUs of Mainit, Alegria and Jabonga, as well as DOH Caraga for facilitating the safe fieldwork and collection of the specimen during the peak of the COVID-19 pandemic. A. Nobleza, A. Bardillas and D. Acido are acknowledged for their assistance in field and laboratory analyses.
Authors’ contributions
J. C. J. supervised the project and secured funding. L. A. E. and J. C. J. performed the laboratory analysis and collection of samples. All authors participated in the writing and approval of the final article.
Financial support
The study was funded by the Department of Science and Technology – Philippine Council for Health Research and Development (DOST-PCHRD) and the Department of Health Caraga (DOH-Caraga) through the Caraga Health Research and Development Consortium (CHRDC).
Competing interest
The authors declare there are no conflicts of interest.
Ethical standards
Before collecting samples, consent from LGUs and farmers was obtained. The collection of fecal samples was non-invasive, as only freshly fallen bovine feces were utilized, hence, ethics clearance was not required.