Na and K are essential nutrients that are needed for the maintenance of total body fluid volume, acid and electrolyte balance and normal cell function. Urinary excretion is the primary mechanism for maintaining body homoeostasis(Reference Young1–3). Dietary Na consumption depends on the cultural context and dietary habits of a population. Na can be found in common table salt and naturally in a variety of foods, such as milk, meat and shellfish. It is often found in high amounts in processed foods such as bread, crackers, processed meats and snack foods. K is commonly found in a variety of unrefined foods, especially fresh fruits, vegetables and beans(4,Reference Brown, Tzoulaki and Candeias5) . Thus, a diet high in processed foods and low in fresh fruits and vegetables is often high in Na and low in K(Reference Wu Leung, Butrum and Chang6–Reference Webster, Dunford and Neal9).
The WHO recommends a Na intake of <2 g/d (equivalent to 5 g salt/d) and intake of K to be at least 3·5 g/d in adults to reduce blood pressure and risk of CVD, stroke and CHD(4,10) . Despite these recommendations, Na intake around the world is more than physiological need. Most adult populations have a mean Na intake of >2·3 g/d(Reference Brown, Tzoulaki and Candeias5,10) . On the contrary, K consumption was less than the recommended level(Reference Van Mierlo, Greyling and Zock11). If an individual consumes Na and K at the levels recommended in the WHO guideline, the ratio of Na:K would be approximately one to one, which is considered beneficial for health(12). However, most populations around the world consume a ratio of Na:K of two to one or more(Reference Elliott13).
Findings from the International Cooperative Study on Salt, Other Factors, and Blood Pressure and the International Study on Macronutrients and Blood Pressure studies indicated a positive association between Na intake and body Na:K ratio and blood pressure in multi-ethnic populations(14,Reference Zhao, Stamler and Yan15) . On the other hand, reduced K consumption has been associated with hypertension and CVD. Appropriate consumption levels could be protective against these conditions(12). The ratio of the two nutrients is an important factor in CVD and mortality(Reference Cook, Obarzanek and Cutler16,Reference Yang, Liu and Kuklina17) . As Na consumption rises, increased consumption of K may be even more beneficial. Because in addition to other benefits, it can mitigate the negative effects of excess Na consumption on blood pressure(Reference Whelton, He and Cutler18).
In 2014, CVD was attributed to 24 % of adult deaths in the Ethiopian capital city Addis Ababa(Reference Misganaw, Mariam and Araya19). Another study reported a 22·6 % prevalence of hypertension in Addis Ababa, while national prevalence was 15·6 %(Reference Abebe, Terefe and Kassahun20).
Two types of knowledge are required for consumers to make informed choices about their intake(Reference Kemm21,Reference Worsley22) . The first is declarative knowledge, also known as knowledge of ‘what is’ (i.e. awareness of things and processes) or ‘know that’ knowledge (e.g. consumers need to know the recommended level of salt intake and the health risks of high salt intakes). The second is procedural knowledge, or ‘know how’ knowledge. Procedural knowledge is about practical skills – how to carry out certain tasks. For example, how to choose a lower salt product by comparing food label information and how to reduce the amount of salt used in the cooking by using herbs and spices(Reference Sarmugam and Worsley23).
The WHO global action plan for the prevention and control of non-communicable diseases identifies a 25 % reduction in premature mortality from CVD, a 25 % reduction in raised blood pressure and a 30 % reduction in mean population salt intake as targets for 2025(24). In Ethiopia, the current K intake level and its ratio with Na were unknown, which plays a great role in achieving these goals. The present study aimed to evaluate Na and K intake and salt-related knowledge, attitude and behaviour among adult populations of Addis Ababa.
Methods
Study area
The study was conducted in Addis Ababa, the capital city of Ethiopia. Addis Ababa is found with an altitude of 2300 m and is located at 901′48′′N with a subtropical highland climate. The city has a total area of 527 km2 and a population density of 5165 km2. The city is organised into three levels of administration: city government, sub-cities and district administrations. Based on the 2007 national Census, Addis Ababa had a total projected population size of 3 243 514 in 2016. Among them, 54 % were females(25). Residents per capita income per year was 1364 US dollars, which was twice that of the national average(26).
According to the national food consumption survey, cereals and legumes are the highest proportion of food groups consumed by women and men in Addis Ababa, accounting for 63·9 % for women and 57·1 % for men(27). The availability of factory processed salt at the household level was estimated at 85 % across the country(28).
Study design
A community-based cross-sectional study design was employed, with the study conducted between March and April 2018.
Sampling and study population
The sample size was calculated using a formula for a single population mean(Reference Gibson and Ferguson29). The following assumptions were employed to calculate the required sample size; mean and sd for K intake at 1·46 g/person per d and 0·8 g/person per d, respectively(Reference Carmelle, Dismand and Corine30), desired degree of precision at 0·12 g/person per d and 95 % of CI, 1·5 design effect and a contingency of 10 % for non-respondent. Consequently, 294 households were adequate for the current study.
A multi-stage stratified sampling technique was applied to recruit study subjects. First, the ten administrative sub-cities were stratified into three groups (least, medium and most developed) based on socio-economic indicators obtained from the 2016 Ethiopian household consumption-expenditure survey(31). We randomly selected one sub-city from each stratum.
Second, the calculated sample size was allocated to the three strata proportional to their number of households. Third, we applied a simple random sampling method to select households using a sampling frame obtained from local authorities. Medically diagnosed hypertension and diabetes mellitus patients were excluded from the study because of the potential alteration on their Na and K intake due to medication and diet restriction.
Data collection
We trained and engaged fourteen female data collectors and three supervisors for the data collection. Four days of training were given to the research team. The training included a briefing about the study, detailed discussion on the questionnaire, anthropometry measurements, dietary data collection, urine sample collection and ethical issues. Small group discussions and demonstrations were part of the training.
Interviewer-based face to face data collection was conducted. During the household visits, the research team explained the aim of the study, and verbal informed consent was obtained before data collection.
Measurements
Socio-demographic and economic characteristics
The socio-demographic and economic status of the study participants were assessed using a questionnaire adopted from Ethiopian Demographic and Health Survey(32).
Twenty-four-hour diet recall
An interactive, multiple-pass 24-h dietary recall questionnaire adapted and validated for use in developing countries(Reference Gibson and Ferguson29) was used to collect dietary intake. In order to precisely estimate the portion size of foods and drinks consumed, we used a food weighing scale, pictures of fruits and vegetables as well as calibrated locally available labelled cups, bowls, spoons and serving spoons. Participants were asked to put an equivalent amount of food they ate when actual food was available in the house. Dietary intake was weighted at the household of the respondent and for shared foods, and the respondents were asked to estimate the portion they ate by using the equipment. Discretionary salt added during eating was asked and estimated using a pinch, salt shaker and food weighing scale. For purchased foods, the price, brand name, and weight or size on the label was recorded together with the number of items consumed.
The dietary recall was repeated in randomly selected 90 households using different interviewers. The repeated data collection was done on a different non-consecutive day than the first to adjust for the day-to-day variation on nutrient intakes(33).
Urine collection
Five-millilitre random urine was collected on site with a labelled additive-free tube. The collected random urine sample from the field was taken to the Ethiopian Public Health Institute clinical chemistry laboratory and refrigerated at 2–8 °C until the next day for analysis.
Anthropometric data
Height and weight measurements were taken to estimate 24-h electrolyte excretion from random urine. We used a locally constructed stadiometer with a precision of 0·1 cm to measure height. The weight of the participants was measured using a digital weighing scale, which has a capacity of 150 kg and a precision of 0·1 kg.
Knowledge, attitude and behaviour about dietary salt
Data on knowledge, attitude and behaviour related to dietary salt among the study population were collected using questionnaires adopted from WHO and other literature(Reference Grimes, Kelley and Stanley34–36). Knowledge questions included the daily maximum recommended intake of salt, sources of salt and the relationship between high salt intake and health problems. Questions to assess attitude included the perception of own salt consumption and perceived importance of lowering salt intake. Salt-related behaviour was evaluated using questions such as behaviour of adding salt and taking actions to lower salt intake.
Data management and analysis
Non-dietary data were checked for completeness and entered into Epi data version 3·1 and exported to Stata 14 software for analysis. The normality of continuous variables was assessed by evaluating histograms, normally distributed data are presented as mean (sd) and skewed data presented as median (interquartile range: 25th–75th), and categorical variables were expressed as a percentage.
Calculating sodium and potassium content of the diet
We used Ethiopian and Tanzanian food composition tables and dietary database of nutri-survey to calculate Na and K content of foods and drinks. Nutrient values of mixed dishes were calculated from recipes, and information on the missing foods was taken from substitutes (estimation from similar foods). Nutrient labelling was used to analyse the nutrient composition of purchased food. The complied food composition table was entered into a software package nutri-survey to create a dietary database and to calculate intake per individual per d. Outliers were checked visually using scatterplot(37). None of the measurements were excluded.
We employed the National Cancer Institute methodology to estimate the usual Na and K intake. The National Cancer Institute methodology was preferred because it employs statistical modelling to account for intake variability. Assumptions of this model include 24-h dietary data are unbiased (but not error-free) and random within- and between-person variation (normally distributed, with a common variance and independent from those of other people). Skewed distributions of intakes were handled with transformations. The method estimates average daily intakes by considering the effect of covariates, which helps to estimate the population mean, usual intake closer to the true value and percentage of the population with usual intakes below/above cut-off points(38,39) .
Urine analysis
The collected urine sample was vortexed, reduced to a sample cup and analysed using Cobas 6000 (c501) analyser. Urinary Na and K were determined by using the ion-selective electrode method, and urinary creatinine was measured by using the enzymatic method.
Estimated 24-h Na and K excretion was calculated from spot urine Na, K and creatinine values adjusted for age, sex, height, weight and BMI using a formula developed by International Cooperative Study on Salt, Other Factors, and Blood Pressure(Reference Elliott, Brown and Dyer40) and Tanaka(Reference Tanaka, Okamura and Miura41).
Salt intake per d was estimated by multiplying Na intake per d by 2·5 according to the WHO recommendation(10).
Prevalence of inadequate potassium intake and excess salt, sodium and sodium:potassium ratio
We estimated the prevalence of inadequate K intake among the study population by comparing intake values with the least recommended value (3·5 g/d). Prevalence of excess Na and excess salt intake was calculated by comparing intake values with the recommended value of 2 g Na/d and 5 g salt/d, respectively. Similarly, the prevalence of excess Na to K intake was calculated by comparing with one to one ratio(4,10) .
Result
A total of 294 households were approached for the study. We had a response rate of 97·3 % (n 286). Two urine samples were discarded because the urine analysing machine could not calculate creatinine and K amount for an unknown reason. Ninety households were interviewed twice for dietary evaluation which results in a total of 386 interviews.
Table 1 shows the socio-demographic characteristics of the respondents. The majority (54·9 %) of the study participants were female. Most of the study participants (83·1 %) were in the age range of 20–39 years, with a median age of 30 years. Two-thirds of the participants (69 %) were married. Family size ranged from 1 to 11 with a median value of 4 per household.
Table 1 Socio-demographic characteristics of study participants in Addis Ababa, Ethiopia, 2018
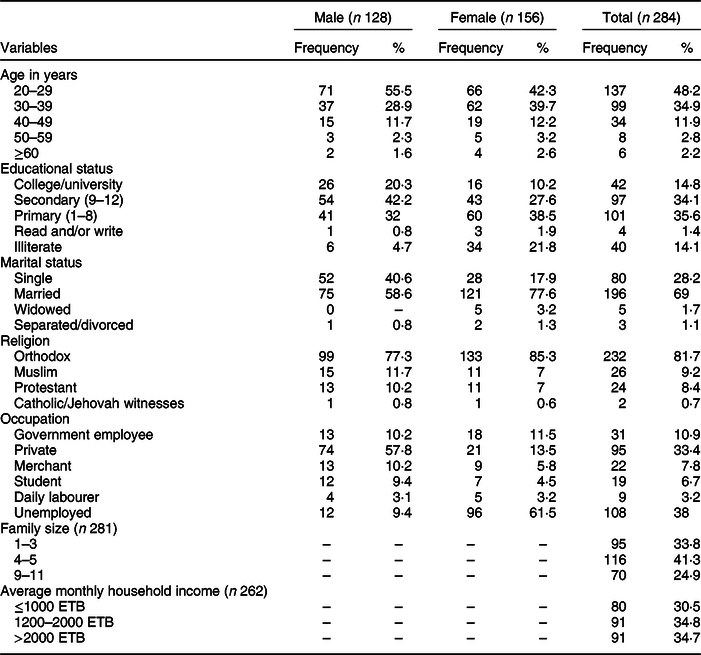
ETB, Ethiopian birr.
Salt, sodium, potassium and sodium:ratio intake measured using 24-h diet recall and urine
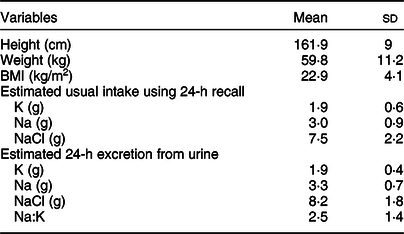
Table 2 shows mean values of anthropometry measurements, salt, Na and K intake estimated from both the diet recall data and spot urine collection. The mean BMI measurement was 22·9 (4·1) kg/m2. Mean Na intake estimated using the diet recall data and urine analysis was 3·0 (0·9) g/d and 3·3 (0·7) g/d, respectively. Besides, the mean K intake estimated using the diet recall data was 1·9 (0·6) g/d, while the mean K intake estimated using 24-h urinary excretion was 1·9 (0·4) g/d. The analysis also showed that the mean Na:K ratio of 2·5 (1·4). The 24-h dietary intake data showed a lower mean salt intake of 7·5 (2·2) g/d than the urine analysis.
Table 2 Salt, sodium and potassium intakes and their ratio measured from 24-h diet recall and spot urine in Addis Ababa, Ethiopia, 2018
Prevalence of inadequate potassium intake and excess salt, sodium and sodium:potassium ratio
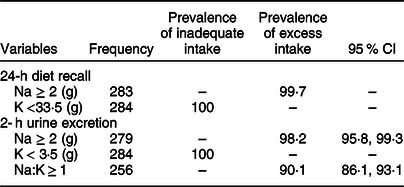
Table 3 shows the prevalence of inadequate K intake and excess intake of salt, Na and Na:K ratio. Based on the 24-h diet recall as well as the spot urine, the daily intake of K was below the recommended amount, i.e. below 3·5 g/d for all study participants. Based on the 24-h diet recall and the spot urine, 99·7 % and 98·2 % of participants had an excess intake of Na, respectively. Also, 90·1 % of the participants had excess Na:K ratio.
Table 3 Prevalence of an inadequacy of potassium and excess salt, sodium and Na:K intake measured from 24-h diet recall and spot urine in Addis Ababa, Ethiopia, 2018
Salt-related knowledge, attitude and behaviour
Table 4 shows the knowledge, attitude and behaviour of study participants about dietary salt. Only five (1·8 %) of participants knew that there is a recommended maximum amount for daily salt consumption. More than half (56·3 % and 58·1 %) of the respondents perceived that their salt intake is ‘just the right amount’ and responded positively to the importance of lowering salt intake, respectively. More than 75 % of the study participants do not add salt at the table and 90·1 % knew about the effect of increased salt consumption on health.
Table 4 Salt-related knowledge, attitude and behaviour of study participants in Addis Ababa, Ethiopia, 2018
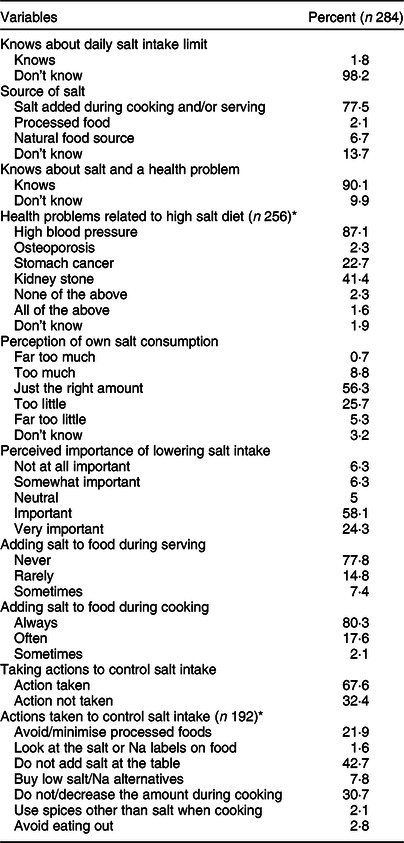
* Multiple answers were possible.
Discussion
This study aimed to estimate the level of K and Na intake and to assess salt-related knowledge, attitude and behaviour among adult populations of Addis Ababa. Face-to-face interview was conducted using standardised questionnaire. Twenty-four-hour diet recall data and urine samples were taken. The result showed a high prevalence of inadequate K intake. Moreover, a high prevalence of excess intake was found for Na and its ratio with K. More than half of the respondents perceived that their salt intake is ‘just the right amount’. The majority of the respondents (80·3 %) added salt during cooking always, and more than two-thirds of the respondents reported that they are taking actions to lower their salt intake.
This study found 3·0 g/d mean consumption of Na estimated from 24-h diet recall data and this is higher than the recommended amount. A consistent finding was reported from a study done in the Cape Verde men population(Reference Daniela, Zélia and Miguel42). Our finding is not consistent with a previous study done in Addis Ababa, which found 4·0 g/d mean Na intake(Reference Abuye, Berhane and Akalu43). This inconsistency may be because we used a different food composition table. Ethiopian food composition table is not complete in quantifying Na and K content of all food types. This forced us to borrow a different country’s food composition table which was available in our respective year of study. Different amount of intake is reported in previous studies done in other countries(Reference Santos, Webster and Land44–Reference Shufa, Ne iman and Batis46). This difference is attributed to the difficulty of quantifying discretionary salt intake, incomplete or/and inaccurate food composition table, demerits of dietary recall difference in intake and diversity of diet.
The current study found a relatively lower amount of Na consumption from diet recall than the urine finding. This was because diet recalls most of the time results in underestimation of intake. Salt/Na intake depends on the amount of food intake especially in foods that undergo some alteration (non-raw foods). Thus, if there is an underestimation of intake, it is likely to get low figures for salt/Na consumption(Reference Freedman, Commins and Moler47,Reference Rhodes, Murayi and Clemens48) .
Na consumption estimated from urinary excretion in the current study was 3·3 g/d. This finding is consistent with a previous study done in the urban settings of Ethiopia, which used a similar method and found 3·3 g/d mean Na consumption. The proportion of the population above the recommended value was 98·2 % and 97·5 % in our study and the previous study, respectively(Reference Feyissa, Yewondwossen and Kissi49). The consistent finding also reported from a study in South Africa, which found 3·3 g/d mean Na intake(Reference Bianca, Schutte and Marike50). A slight difference is observed in the prevalence of excess Na intake in Benin. This may be because the study done in Benin included urban and rural parts of the country(Reference Carmelle, Dismand and Corine30). A systematic review conducted in sub-Saharan African countries reported that salt/Na intake in the rural setting is lower than in urban settings. This can be explained by westernisation of cities and towns. Because salty packed foods and fast foods high in salt are more accessible in urban areas than in rural areas(Reference Oyebode, Oti and Chen51). Inconsistent higher findings also reported from China, this may be explained by their culture of consuming salty sauces besides the use of cooking salt(Reference Shufa, Ne iman and Batis46,Reference Liuxia, Xiaolei and Huicheng52) .
K intake from 24-h diet recall in our study was 1·9 g/d, and none of the intakes were adequate. This finding is much lower than the least daily recommended amount. It is because in Ethiopia the consumption of fruit and vegetables is very low. We found that only two participants (0·7 %) had adequate fruit and vegetable intake, which are the main sources of K. Another study found a 0·9 % prevalence of fruit and/or vegetable consumption in urban areas of Ethiopia(Reference Terefe, Kassahun and Tefera53). This might be because the cultivation of fruits and vegetables in Ethiopia was sub-optimal(Reference Tsegaye, Ahmed and Dilnesaw54). A relatively higher intake of K was reported from the Cape Verdean study. This was because their daily consumption of fruits and vegetables was higher than our study(Reference Daniela, Zélia and Miguel42). A 3·1 and 3·2 g/d mean K intake was reported from South Korea for the age group of 19–39 years and 40–59 years, respectively, they justify that this intake comes from white rice, kimchi, vegetable and fruits, which are not common foods in the Ethiopian setting(Reference Haeng-Shin, Duffey and Popkin55). A similar finding was reported from China which used three consecutive 24-h diet data(Reference Shufa, Ne iman and Batis46).
Our study reported 1·9 (0·4) g/d mean K excretion, and all values were below the recommended amount. A similar finding is reported from Mozambique 1·9 (0·8) g/d with an inadequate prevalence of 96·3 %(Reference Ana, Damasceno and Neusa56). Similar findings to our study were also reported from existing literature(Reference Liuxia, Xiaolei and Huicheng52,Reference Keiko, Ken and Yuki57–Reference Samuel, Adewole and Solomon59) . Relatively higher K intake was reported from the sub-Saharan country Benin, this may be due to the inclusion of rural communities in the study. Hence, the availability of fresh fruits, vegetables and beans is relatively high in the rural area especially if there is home gardening. Another explanation could be as participants in Benin’s study were told to abstain from going to work and to stay in health care centres on the day of urine collection. This might have altered the participants’ dietary habits(Reference Carmelle, Dismand and Corine30). The K intake reported from New Zealand was higher than the current study; this can be explained because they did not exclude hypertensive patients. Even though they did not mention the type of medication they took, some hypertensive drugs alter the K excretion(Reference Rachael, Julia and Sheila60).
The mean consumption of Na:K ratio was 2·5 (1·4) g/d. The prevalence of excess intake was 90·1 % compared with a one to one ratio. Consumption of Na and K at one to one ratio is considered to be beneficial for health(12). Our finding was consistent with previous studies(Reference Shufa, Ne iman and Batis46,Reference Keiko, Ken and Yuki57) . However, inconsistent findings were also reported from existing studies(Reference Carmelle, Dismand and Corine30,Reference Haeng-Shin, Duffey and Popkin55,Reference Rachael, Julia and Sheila60) . This inconsistency is attributed to the above-mentioned reasons for each nutrients difference in intake, which affects the ratio.
Our study finding showed a higher Na:K ratio which puts the population at risk of hypertension and CVD. The ratio of the two nutrients is more influential than the effect of each nutrient independently. It has been suggested that individuals with diets that are low in K are particularly vulnerable to the hypertensive effects of a high Na intake. In evaluating results, a high amount of Na finding only may not be as severe as high Na:K ratio. Because a high Na intake might be accompanied by a good K intake which gives a lower ratio of the two nutrients(Reference Cook, Obarzanek and Cutler16–Reference Whelton, He and Cutler18,Reference Kawasaki, Itoh and Kawasaki61–Reference Perez and Chang64) .
Knowledge of study participants on maximum daily salt consumption (5 g/d) was much lower than other country studies(Reference Zhang, Xu and Ma65,Reference Nasreddine, Akl and Al-Shaar66) . Since there was nothing done in the study setting to create awareness about dietary salt consumption, it is likely to get a lower figure than the others. Nine out of ten individuals knew that a high salt diet could cause a serious health problem. Among them, majorities mentioned blood pressure. This finding is comparable with published studies(Reference Grimes, Kelley and Stanley34,Reference Johnson, Mohan and Rogers35,67) .
A consistent finding was reported on the perception of own salt intake to be ‘just the right amount’(Reference Rajib, Rajib Chandra and Palash Chandra68) and too much or far too much(67). Though we found a high prevalence of excess salt intake, a lower proportion of the population believed that their intake was high. This finding was not in concordance with previous studies(Reference Grimes, Kelley and Stanley34,Reference Zhang, Xu and Ma65,Reference Nasreddine, Akl and Al-Shaar66,Reference Magalhaes, Sanhangala and Dombele69) . The potential reason might be due to the presence of salt reduction initiation in other countries’ study.
The majority of participants agreed on the importance of lowering salt intake. As a result, 67·7 % took actions to lower their intake. This finding was higher than the previous study done in Ethiopia. Actions taken to lower salt intake in the two studies were somehow different. This may attribute to the variation in the study setting in which the current study included only the urban population(67).
Our finding on salt usage during cooking was consistent with a previous study in Ethiopia. However, there was a discrepancy in salt usage at the table before eating. This could be because the previous study included salty sauce usage besides the discretionary salt added at the table, which was not included in the current study(67). Our finding was consistent with the study finding of Angola and India, and the majority of participants stated that salt was always added in preparing food at home(Reference Johnson, Mohan and Rogers35,Reference Magalhaes, Sanhangala and Dombele69) .
The present study shall be interpreted with the following strengths and limitations. In this study, discretionary salt usage was quantified which helps us to get a good figure on the actual salt intake. A combination of biochemical and dietary assessment was used to assess Na and K intake, which decreases the effect of measurement error. We used the National Cancer Institute method to estimate the usual intake. This method considers factors affecting intake, which is helpful to have an estimate closer to the true value. Moreover, all days of the week were represented equally during data collection to adjust for day-to-day variation in nutrient intake. However, this study has limitations that need to be acknowledged. First, we used self-reported dietary data, which may be different from actual behaviour and may result in most of the time in underreporting. To overcome this problem, we used pictures and food weighing scales during portion size estimation and respondents were probed for snacks, fruits and outdoor consumption. Second, the Ethiopian food composition table is not complete regarding Na and K values of diet. This forced us to borrow values from another country’s food composition table which decrease reliability of the dietary database. Third, we used spot urine to estimate Na and K consumption. It is known that spot urine analysis does not give a stable estimate at the individual level. But it was proved that it can provide a reliable estimate at the population level. Though validation studies support the use of spot urine at the population level, they also showed there could be an underestimation(Reference Tanaka, Okamura and Miura41,Reference Bianca, Schutte and Marike50) . Fourth, the formula we used to estimate 24-h electrolyte excretion from spot urine was not validated in our country. Even though it is not validated in our country, we used formulas which had a relatively good result during validation studies among heterogeneous population including Africa.
Conclusion
In conclusion, we found a high prevalence of inadequate K intake and a high prevalence of excess Na intake. The ratio of the two nutrients is higher than what is recommended to be beneficial for health, which put the population at risk of hypertension and CVD. Salt-related knowledge, attitude and behaviour were found to be low as many participants were unaware of their high salt intake. Thus, an intervention targeting to decrease Na intake and increase K intake is needed. Decreasing salt consumption and increasing fruits and vegetable consumption could be an effective way. Because salt and fruits and vegetables are the main sources of Na and K, respectively.
Acknowledgements
Acknowledgements: We would like to thank Mr Demewoz Haile, Mr Feyissa Chala, Mr Masresha Tesema and Mr Meseret Werede for their technical support. We would like to extend our compliment to data collectors and study participants. We would not have done this without you. Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: There are no conflicts of interest. Authorship: S.M.S. initiated the research, prepared the proposal, conducted the research, entered the data, analysed the data, interpreted the findings and wrote the manuscript. S.H.G. and B.S.E. were involved in initiating the research, interpreting the findings and reviewing the manuscript. B.N.O. and G.A.B. were involved in urine sample analysis. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guideline laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Research Ethical Committee of School of Public Health, Addis Ababa University and Addis Ababa regional health bureau. Written informed consent was obtained from all subjects.









