Iron deficiency (ID) is the most common nutritional disorder worldwide, affecting more than 2 billion people globally. Iron-deficiency anaemia (IDA) is highly widespread in developing countries but also remains a problem in developed countries. Adolescents are tomorrow’s adult population and their health and well-being are crucial( Reference Shekhwat, Gupta and Gupta 1 ).
Managing anaemia is one of the global health goals. Many adolescents are suffering from ID with adverse effects on their health( 2 ). Anaemia and IDA are indicators of both poor nutrition and poor health, and have been associated with cognitive impairment including reduced attention span, intelligence and sensory perception functions( Reference Bhanushali, Shirode and Joshi 3 , Reference Jáuregui-Lobera 4 ).
Several risk factors may contribute to cause ID and IDA during adolescence, including sociodemographic and dietary factors( Reference Falkingham, Abdelhamid and Curtis 5 ). ID can incur neurocognitive impairment leading to psychomotor and cognitive abnormalities in children and adolescents without anaemia. IDA raises child mortality rates and susceptibility to infectious diseases and delays child development. Causes of ID or IDA include inadequate nutrition of Fe intake, gastrointestinal malabsorption and chronic blood loss( Reference Huang, Tok and Lu 6 ). Prevalence of anaemia was found to be lower in nuclear families than in extended families( Reference Gupta and Kochar 7 ).
One of the major risk factors affecting IDA is unhealthy dietary habits( Reference Jeong, Park and Hong 8 ). The availability of Fe depends on its absorption rate. According to the National Institutes of Health, overall 10–15 % of dietary Fe is absorbed by healthy adults. The body absorbs 15–35 % of haem Fe that comes from animal sources, and this is not significantly influenced by other nutrients in the diet. In contrast, the body absorbs only 2–20 % of non-haem Fe that comes from plant sources( Reference Hurrell and Egli 9 ). This is because the body has to alter the non-haem Fe during digestion in order to utilize it fully. The intake of all animal food sources like meat, fish, poultry and others increases with household income, thus household income influences Fe intake.
The present study was set up in the Gaza Strip, Palestine, due to some alarming factors. In 2009, the Gaza Strip witnessed a series of dramatic events which were pivotal in the Arab–Israeli conflict. The Applied Research Institute Jerusalem reported that Israel tightened the blockade on the Gaza Strip, depriving people of essential raw materials (flour, sugar, oil, rice and salt) and medicines( 10 ). According to the Palestinian Central Bureau of Statistics in 2018, 53·0 % of the population in the Gaza Strip was found to be poor in 2017. Unemployment in Palestine is a dangerous phenomenon that many Palestinians suffer from. Since the Israeli occupation imposed the siege, the cross-border closed and the Palestinian economy was destroyed as well, so most Palestinian workers and university graduates suffer from destructive unemployment( 11 ).
The present study was undertaken to identify the prevalence of anaemia, ID and IDA, well as risk factors associated with ID and IDA, among female adolescents in the Gaza Strip, Palestine.
Methods
Study design
The present study was conducted using the quantitative descriptive method with a cross-sectional design. Female adolescents aged 15–19 years in secondary schools of the Gaza Strip, Palestine, were enrolled randomly for the study. Three hundred and thirty adolescent female students were required for the study, this sample size being calculated using a formula( Reference Charan and Biswas 12 ) with an estimated anaemia prevalence of 0·30, prevalence of non-anaemia estimated at 0·70 and with 0·05 margin of error. The 330 students were selected from the list of names records of school classes based on eligibility criteria.
The data were collected using an FFQ and sociodemographic, sedentary behaviour and physical activity questionnaires. In addition, anthropometric measurements and blood analyses were conducted. A pilot study was conducted for the questionnaires that were written in Arabic language.
Study population
The population in the study included all the Palestinian female adolescents enrolled in secondary schools in academic years 2015–2016. Five female secondary schools were selected randomly from five governorates of the Gaza Strip. Within each school, classes were chosen randomly.
Eligibility criteria
The inclusion criteria were female respondent aged 15–19 years and having stayed in the Gaza Strip for more than 1 year. The exclusion criteria were female respondent with a history of chronic diseases, any blood diseases, married, pregnant, lactating, with disabilities and receipt of Fe supplements within the past month.
Sampling methodology
A stratified multistage cluster sampling technique was used to obtain the sample for the study. As there are five governorates in the Gaza Strip, five schools were adequate to obtain the necessary sample size from a total of 145 schools. A minimum number of forty-nine and a maximum number of 137 among the female adolescents in the 10th, 11th and 12th grades were selected from each school. The questionnaires were distributed and monitored along with the filling-in process.
Study tools and instruments
Three types of questionnaires were used in the present study: (i) FFQ; (ii) sociodemographic and sedentary behaviour; and (iii) physical activity pattern.
FFQ have been developed to be easy to complete, analysable by computer and inexpensive. The FFQ was derived from the National Institutes of Health and translated into the Arabic language, with reliability and validity established by Tayyem and colleagues( Reference Tayyem, Abu-Mweis and Bawadi 13 ). The FFQ included ninety-six food items and needed about 20–25 min to complete. It was used to collect qualitative, descriptive information regarding the food habits of the female adolescents during the past 12 months.
The present study used the physical activity questionnaire designed by the Arab Teens Lifestyle Study (ATLS) to evaluate the physical activity pattern among adolescents( Reference Al-Hazzaa 14 ). The questionnaire included thirty-three questions addressing the frequency, duration and intensity of a variety of light, moderate and vigorous physical activities during a usual week. The questionnaire also included transport, household, fitness and sport activities.
Anthropometric measurements, height and weight with minor variables (i.e. birth date), were used. Body weight was measured using a calibrated scale (Seca model 750, Germany) and height was measured with a stadiometer (Seca body meter 206, Germany). BMI-for-age was used in the present study, computed using the WHO AnthroPlus software program version 7.1.5.2 for age 5–19 years to monitor the growth of school-age adolescents. Cut-off values used for classification of the anthropometric indicator were those according to the AnthroPlus software( 15 ).
Blood parameters were assessed from non-fasting blood samples( 16 ). Anaemia status was assessed by measuring Hb concentration using analysis of complete blood count (Horiba ABX Micros ES 60, France). Anaemia and ID were defined according to WHO guidelines as Hb<12·0 g/dl and ferritin<15 µg/l, respectively. Fe status was assessed by measuring serum ferritin, which was determined using a model BS-120 chemistry auto analyser (Shenzhen Mindray Bio-medical Electronic Co. Ltd). According to serum ferritin concentration, Fe status can be divided into three groups: ID, borderline Fe depletion and Fe replete( Reference Grondin, Ruivard and Perreve 17 ).
Ethical considerations
Prior to the data collection, written permission to carry out the study was obtained from the Helsinki Committee and the Ministry of Education in Palestine. After reviewing the study protocol, the Human Ethics Committee of Universiti Kebangsaan Malaysia gave its ethical approval (reference number UKM1.5.3.5/244/NN-025-2015).
Statistical analysis
The statistical software package IBM SPSS Statistics version 22 was used for analysis of data, which was conducted by review of the filled-in questionnaires, coding the questions, data entry, data cleaning and coding variables. All data were analysed using descriptive and inferential analysis.
1. Test of normality: Kolmogorov–Smirnov statistics were used to examine the normality of the data distribution for each variable, with a P value of less than 0·05 indicating that the normality assumption of the data was violated. The Hb data were normally distributed; thus Hb data were presented as means and sd. Serum ferritin concentrations were skewed towards higher values; thus medians and interquartile ranges (IQR) were used as the measure of central tendency. Non-parametric tests were used when the normality assumption was violated.
2. Descriptive statistics: means and sd were used to describe the continuous data while numbers and percentages were used to describe the categorical data. Median and IQR were used for continuous data when the distribution was not normal.
3. Univariable analysis: univariable analysis was conducted to select potential independent variables associated with ID and IDA using the independent t test and simple logistic regression. The independent t test was used for comparison of mean difference in scores. However, the Wilcoxon signed-rank test was used if the normality assumption was violated. Spearman’s coefficient (ρ) was used to investigate the correlation between ferritin and Hb and other independent variables. Simple logistic regression was used to examine the potential association between the factors that affected Hb and ferritin levels.
4. Multivariable analysis: one of the specific objectives of the study was to predict the risk factors of anaemia, ID and IDA. Therefore, logistic regression analyses were carried out to determine the factors associated with anaemia, ID and IDA in female adolescents aged 15–19 years. The independent variables with P<0·25 and logically related to the effect on Hb and ferritin levels that were tested in simple logistic regression were then included in the multivariable analysis (multiple logistic regression).
Results
Sociodemographic characteristics of the female adolescents
Table 1 shows the distribution of age, height, weight and BMI-for-age of the respondents. The overall mean age of 330 respondents was 16·37 (sd 0·86) years and ranged from 15·0 to 18·7 years. Moreover, the mean weight of the female adolescents was 56·71 (sd 10·99) kg with a range of 37·4–101·7 kg. Besides, the mean height of the respondents was 159·59 (sd 5·77) cm, while the mean BMI-for-age was 22·25 (sd 3·87) kg/m2.
Table 1 Anthropometric characteristics of the female adolescents (n 330) aged 15–19 years, Gaza Strip, Palestine, academic years 2015–2016
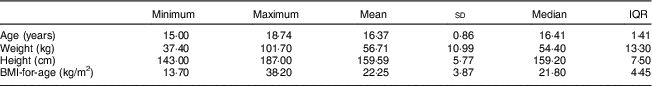
IQR, interquartile range.
Haematological and biochemical characteristics of the female adolescents
All female adolescents underwent blood tests, namely complete blood count, ferritin, high-sensitivity C-reactive protein (hs-CRP) and erythrocyte sedimentation rate, as shown in Table 2. The overall median ferritin level in the female adolescents was 19·0 (IQR 11·00–29·3) μg/l. Moreover, the median Hb of the adolescents was 12·2 (IQR 11·6–12·9) g/dl with a range from 8·5 to 14·3 g/dl. Meanwhile, the median of hs-CRP and erythrocyte sedimentation rate were 1·6 mg/l and 15·0, and ranged from 0·1 to 94·0 mg/l and from 2·0 to 65·0, respectively.
Table 2 Blood biochemistry parameters of the female adolescents (n 330) aged 15–19 years, Gaza Strip, Palestine, academic years 2015–2016

IQR, interquartile range; MCHC, mean cell Hb concentration; MCV, mean cell volume; RDW, red cell distribution width; hs-CRP, high-sensitivity C-reactive protein; ESR, erythrocyte sedimentation rate.
Prevalence of anaemia, iron deficiency and iron-deficiency anaemia in the female adolescents
Additionally, the prevalence of anaemia (Hb<12·0 g/dl) among the female adolescents aged 15–19 years was 35·8 %, as shown in Table 3. The prevalence of ID (ferritin<15·0 μg/l) observed among the study sample was 40·3 %. This showed that ID was quite high among the female adolescents, namely in almost half of the respondents. On the other hand, the prevalence of IDA (Hb< 12·0 g/dl and ferritin<15·0 μg/l) was 26·0 %. Thus, Fe was not the only risk factor contributing to anaemia among the respondents. Table 3 also shows that the percentage of ID with acute-phase reactant was 53·0 % (hs-CRP>10 mg/l + ferritin<50 µg/l).
Table 3 Prevalence of anaemia, iron deficiency (ID) and iron deficiency anaemia (IDA) among the female adolescents (n 330) aged 15–19 years, Gaza Strip, Palestine, academic years 2015–2016
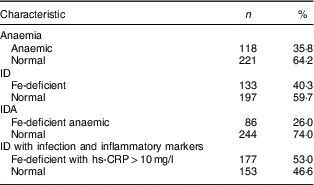
hs-CRP, high-sensitivity C-reactive protein.
Correlations between ferritin and Hb levels and other variables
Spearman correlation was carried out to determine the relationships between food consumption, physical activity, sedentary lifestyle behaviours and blood parameter factors which were involved in the ferritin and Hb levels of the female adolescents.
The dependent variable of ferritin level was significantly correlated with different parameters including consumption of vegetables, fruits, meat and meat products, grains, legumes, milk and milk products, beverages, and desserts and snacks. Similarly, there was a significant correlation with hs-CRP, mean cell volume, mean cell Hb concentration and red cell distribution width, as demonstrated in Table 4. Table 4 also demonstrates correlates of the dependent variable of Hb level: there was a significant correlation with different parameters including consumption of fruits, meat and meat products, grains, legumes, milk and milk products, beverages, and desserts and snacks; as well as with erythrocyte sedimentation rate, mean cell volume, mean cell Hb concentration and red cell distribution width.
Table 4 Correlation of ferritin and Hb levels with other variables among the female adolescents (n 330) aged 15–19 years, Gaza Strip, Palestine, academic years 2015–2016
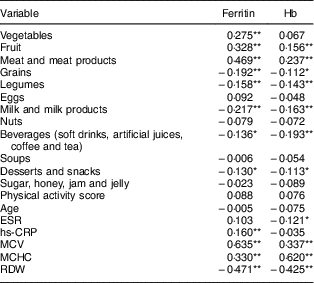
ESR, erythrocyte sedimentation rate; hs-CRP, high-sensitivity C-reactive protein; MCV, mean cell volume; MCHC, mean cell Hb concentration; RDW, red cell distribution width.
Spearman correlation: *P<0·05, **P<0·01.
Associations between Hb and FFQ and blood parameters by multiple logistic regression
Table 5 presents all determinants appearing in the logistic regression model that remained significantly associated with anaemia status, which are meat and meat products, milk and milk products, and beverages (soft drinks, artificial juices, coffee and tea). The overall status of the model was significant (constant of significance=0·097). Multivariate analysis showed that decreased meat consumption was more likely to lead to anaemia (OR=0·95, 95 % CI 0·92, 0·96, P <0·001). Moreover, milk and drinks were significantly associated with anaemia (OR=1·05, 95 % CI 1·01, 1·07, P=0·004 and OR=1·05, 95 % CI 1·01, 1·07, P=0·003, respectively).
Table 5 Food item factors associated with anaemia, by multiple logistic regression analysis, among the female adolescents (n 330) aged 15–19 years, Gaza Strip, Palestine, academic years 2015–2016
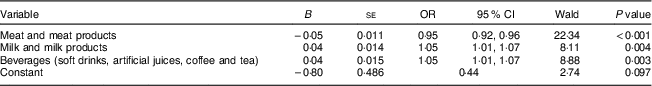
Associations between ferritin and FFQ and blood parameters by multiple logistic regression
Table 6 shows all determinants appearing in the logistic regression model that remained significantly associated with ID status, namely vegetables, fruits, meat and meat products, legumes, and milk and milk products. The overall status of the model was significant (constant of significance=0·005) and Nagelkerke R 2 was 0·430, which means that 43 % of the variation in the outcome (ferritin) was explained by this logistic regression model. Decrease in vegetable, fruit and meat consumption was more likely to lead to ID (OR=0·98, 95 % CI 0·96, 0·99, P=0·011; OR=0·98, 95 % CI, 0·96, 0·99, P=0·036; and OR=0·91, 95 % CI, 0·88, 0·94, P <0·001, respectively). Besides, milk and legumes were significantly associated with ID (OR=1·06, 95 % CI 1·02, 1·10, P=0·003 and OR=1·09, 95 % CI 1·05, 1·13, P<0·001, respectively).
Table 6 Food item factors associated with iron deficiency, by multiple logistic regression analysis, among the female adolescents (n 330) aged 15–19 years, Gaza Strip, Palestine, academic years 2015–2016

Associations between Hb and other variables by using multiple logistic regression
Table 7 presents all determinants appearing in this model that remained significantly associated with ID status, namely eating breakfast, eating lunch, eating late at night and beverages. The overall status of the model was significant (constant of significance <0·001). Irregular breakfast, not taking breakfast and skipping lunch were more likely to lead to anaemia (OR=2·53, 95 % CI 1·30, 4·96, P=0·006; OR= 3·64, 95 % CI 1·56, 8·48, P=0·003; and OR 9·73, 95 % CI 1·15, 82·18, P=0·037, respectively) compared with those who regularly took breakfast or regularly took lunch.
Table 7 Factors associated with anaemia, by multiple logistic regression analysis, among the female adolescents (n 330) aged 15–19 years, Gaza Strip, Palestine, academic years 2015–2016

Ref., reference category.
*Significant at P ≤ 0·05.
Interestingly, intake of large size beverages among the participants was significantly associated with anaemia (OR=5·48, 95 % CI 1·84, 16·35, P=0·002).
Associations between ferritin and other variables by using multiple logistic regression
Lastly, Table 8 presents two types of model: the first model is a menstruation status and dietary behaviour model, while the second model is a blood parameters model. The overall status of the first model was significant (constant of significance <0·001) and the second model was also significant (constant of significance=0·010).
Table 8 Factors associated with iron deficiency, by multiple logistic regression analysis, among the female adolescents (n 330) aged 15–19 years, Gaza Strip, Palestine, academic years 2015–2016

Ref., reference category; MCV, mean cell volume; RDW, red cell distribution width.
*Significant at P ≤ 0·05.
Table 8 presents all determinants appearing in these models that remained significantly associated with ID status, namely menstrual period, mother’s education, eating breakfast, eating late at night and beverages. First to observe from Table 8 is that ferritin level was negatively and significantly associated with the number of days of the menstrual period (OR=7·23, 95 % CI 2·37, 21·98, P<0·001). Moreover, by analysis of multivariate regression, the negative association between mother’s education and ferritin level could be confirmed (OR=1·88, 95 % CI 1·08, 3·25, P=0·024). Irregularity and skipping of breakfast were more likely to lead to ID (OR=3·10, 95 % CI 1·55, 6·17, P=0·001 and OR=4·01, 95 % CI, 1·72, 9·33, P=0·001, respectively), while intake of large size beverages was also significantly associated with ID (OR= 7·21, 95 % CI 2·55, 20·32, P<0·001).
In addition, ferritin level was statistically and negatively associated with mean cell volume (OR=0·82, 95 % CI 0·77, 0·86, P<0·001), as shown in Table 8. Meanwhile, a positive association emerged between ID and increasing red cell distribution width (OR=1·76, 95 % CI, 1·33, 2·31, P<0·001).
Discussion
Food security has evidently become worse in the Gaza Strip, Palestine, since the siege started in 2007 and furthermore immediately after the Israeli military invasion in December 2008. In fact, food insecurity is associated with decreased nutrient intakes and poor health, which can lead to ID and IDA. The intended aims of the present study were to establish the prevalence of anaemia, ID and IDA among female adolescents aged 15–19 years in the Gaza Strip, Palestine, and to analyse the associated risk factors.
Sociodemographic factors
Sociodemographic information obtained from the questionnaire included age, mother’s education, father’s education and house location. However, the present study did not include information on household income because it is often not the best indicator of socio-economic status; the use of household income does not address the fact that family members may have unequal access to household income( 18 , Reference Liu, Ai and Hanlon 19 ).
The prevalence of ID (ferritin<15 µg/l) was 40·3 % and the prevalence of IDA (Hb<12 g/dl and ferritin<15 µg/l) was 26·0 %. ID prevalence in the current study (40·3 %) was much higher than the estimates in most developed countries (Canada 6 %, Kazakhstan 17·1 %, USA 16 %, Spain 6·7 %, England 4 %)( 20 – Reference Nelson, White and Rhodes 25 ). Compared with developing countries, ID prevalence in the current study was lower than the prevalence among Malaysian female adolescents aged 12–19 years and 7–12 years (49·5 and 59·1 %, respectively)( Reference Foo, Khor and Tee 26 , Reference Ngui, Lim and Chong Kin 27 ). A study in Bangladesh indicated that 17 % of the female adolescents had ID( Reference Ahmed, Khan and Islam 28 ). Among the Middle Eastern countries, prevalence of anaemia among female adolescents was reported to be 21·8 % in Saudi Arabia, 43·2 % in Kuwait( Reference Abalkhail and Shawky 29 ) and 46·6 % in Egypt (age 10–19 years)( Reference Barduagni, Ahmed and Curtale 30 ). In contrast to Africa, our study showed a lower level of anaemia among female adolescents. Overall, in the Gaza Strip, ID was highly prevalent among anaemic female adolescents: 72·6 % of anaemic females were Fe deficient. Therefore, the results suggest that ID is a major contributor to anaemia, but other causes should also be considered in the population.
The association of anaemia with different factors, including the female adolescents’ maternal and paternal educational levels, was assessed considering socio-economic and demographic factors. From all studied factors, age was associated with anaemia and mother’s education level was associated with ID. The study did not find an association between mother’s education and incidence of anaemia (P>0·05), but an association was found between high maternal education level and incidence of ID (P<0·05). Mother’s educational level was correlated with dietary mineral and vitamin intakes and with the quality of diet among adolescents in Spain( Reference Tur, Puig and Benito 31 ). Furthermore, it was found that mother’s educational level influenced the intakes of meat, fish, fruits and vegetables among Spanish children and adolescents( Reference Serra Majem, Ribas Barba and Pérez Rodrigo 32 ). In Malaysia, low mother’s education was found to be a significant risk factor for IDA( Reference Al-Mekhlafi, Surin and Atiya 33 ). Thus, female adolescents whose mothers had less than secondary school level of education had increased the risk of ID compared with female adolescents whose mothers had secondary school level of education or above.
Dietary habits
One of the major risk factors of IDA is unhealthy dietary habits. Unhealthy dietary habits frequently observed among adolescents include changing the main meals for snacks, skipping breakfast, reducing the intake of fruits and vegetables and increasing the consumption of junk foods (‘empty-calorie’ sugary foods and sodas/soft drinks)( Reference Bagni, Luiz and Veige 34 ). Nutritional ID increases when physiological requirements cannot be met by absorption of Fe from the food ingested( Reference Zimmermann and Hurrell 35 ). Many people are dependent on plant-based foods from which Fe absorption is poor and several substances in the diet may interfere with this. Major findings in the current study were that a large proportion of female adolescents reported unhealthy dietary habits including skipping breakfast and making less choices of healthy foods.
Studies in several Arab countries have revealed that the dietary habits of adolescents are characterized by high intake of foods rich in fat and energy, as well as salt and sugar( Reference Tayyem, Al-Hazzaa and Abu-Mweis 36 ). However, a recent study conducted in seven Arab countries (Palestine, Jordan, Syria, Kuwait, United Arab Emirates, Libya, Algeria) found that lack of knowledge on healthy eating and lack of motivation to eat a healthy diet were the main barriers to healthy eating among adolescents( Reference Musaiger, Al-Mannai and Tayyem 37 ). Moreover, the knowledge regarding IDA among the participants was low( Reference Jalambo, Naser and Sharif 38 ). Morning snacks between breakfast and lunch were skipped by 24·7 % of female adolescents in the current study, compared with 23·0 % who skipped snacks between lunch and dinner. Moreover, these findings are consistent with a study in Syria where the morning snack between breakfast and lunch was skipped by 25·1 % of Syrian adolescent students, compared with 32·1 % who skipped an afternoon snack( Reference Musaiger and Kalam 39 ). In contrast, the morning snack between breakfast and lunch was skipped by 24·7 % of Sudanese female adolescents, compared with 50·0 % who skipped an afternoon snack( Reference Musaiger, Nabaj and Al-Mannai 40 ).
Besides, the busy lifestyle of today’s families results in adolescents skipping meals or relying on snack foods for basic nutrition( 41 ). The meal patterns of adolescents are becoming more disorganized. Some of their dietary patterns are energy-dense foods, which are low in Fe, Ca, vitamin A, folic acid and fibre( 42 ). Eating patterns such as snacking, skipping breakfast, dieting and adoption of specific diets are the most common features among adolescents that could cause diet-related illness later in life( Reference Al Sabbah, Vereecken and Kolsteren 43 ).
Sedentary behaviours
The findings of the present study indicated that emotional eating is not only widespread in developed countries, but also in developing countries like Palestine. This may due to the dietary transition, which is happening in poor places in Palestine. The most common types of emotional eating among female adolescents were eating while watching television and eating late at night. In addition, previous studies reported that these two eating habits were associated with obesity. The current study found that most female adolescents (64·8 %) engaged in physical activity at home, while (17·3 %) engaged in physical activity at school with their friends.
The majority of the female adolescents in the current study exceeded recommendations for the time spent watching television and using a computer. Thus, watching television, computer and Internet use were additional factors that may contribute to sedentary behaviours among female adolescents. The total time spent watching television and using the Internet was a factor related to increased sedentary behaviours and consequently decreased physical activity( Reference Tremblay, LeBlanc and Kho 44 ).
Physical activity patterns
The present study results showed that there was no association of ID and anaemia with the practice of physical activity (P>0·05). A recent study conducted in Japan also reported that there was no significant correlation between physical activity and ID compared with non-iron deficient female adolescents aged 17–18 years( Reference Kokubo, Ohira and Tada 45 ).
The international consensus conference on physical activity guidelines for adolescents recommended that all adolescents should be physically active daily, or nearly daily, as part of play games, works, sports, physical education, transportation, recreation or planned exercise( Reference Sallis, Patrick and Long 46 ). According to the physical activity practice among female adolescents, the overall prevalence of inactive, minimal active and active among female adolescents in the current study was 25·9, 66·5 and 7·6 %, respectively. This indicated that they practised only a little or no physical activity. Besides, female adolescents in all age in groups in the Gaza Strip were not involved in sports clubs. Hence, adolescents have the tendency to live a sedentary lifestyle that may be due to the customs and traditions in Palestinian society itself where females usually stay at home when they enter puberty.
Strengths and limitations
The current study used haematological and biochemical tests (complete blood count, hs-CRP, erythrocyte sedimentation rate and serum ferritin) to identify IDA. Additionally, the presence of hs-CRP assessment was a strength to avoid overestimation or underestimation. Thus, the precise level of ID in the studied female adolescents could be elucidated.
The present study determined the prevalence of anaemia, ID and IDA. In contrast, other studies determined just the prevalence of anaemia among adolescents( Reference Jalambo, Hamad and Abed 47 ). Serum ferritin testing was conducted for the entire study sample (n 330), which is not common in the previous local studies among the same age group in the Gaza Strip due to its high cost. The study’s target group was female adolescents, who are at high risk of IDA.
There are also several limitations regarding the current study. It was conducted in the Gaza Strip only and therefore does not represent Palestine. Most of the data used in the study were from one phase (cross-sectional study) and reported by the female adolescents. This may create biases due to incomplete responses and the response relies on memory. The data in the cross-sectional study were based on self-report questionnaires, except anthropometric and blood parameter measurements. Although the researchers made an effort to minimize any possible biased reporting by the female adolescents during filling out the questionnaires, self-administered questionnaires used to assess dietary habits among female adolescents tend to result in reporting errors and self-administered physical activity often results in an overestimation.
Furthermore, socio-economic status was not assessed in the present study since the majority of the female students did not know their family’s monthly income and expenditure. Conversely, the data provided by the current study add to the limited existing data on lifestyle and dietary habits of Palestinian female adolescents. The FFQ was based on semi-quantitative information and thus could not estimate the precise nutrient intakes.
Conclusion
In conclusion, the current study has shown that the prevalence of anaemia and ID among female adolescents aged 15–19 years in the Gaza Strip, Palestine, was 35·8 and 40·3 %, respectively. Female adolescents in the Gaza Strip seem to be moving towards unhealthy dietary habits. A significant association was found between ID, anaemia and IDA and dietary habits including skipping breakfast and meal size of junk foods like beverages (soft drinks, artificial juices, coffee and tea). Also, there were associations between low consumption of fruits and vegetables and IDA in the female adolescents. Moreover, factors such consumption of milk and legumes also made the female adolescents prone to ID and IDA.
Another factor affecting anaemia and ID was excess menstrual blood loss. Moreover, a statistically significant association was found between mother’s education and ID but not with the other sociodemographic factors. Besides, a statistical association was found between long menstrual period and anaemia.
Acknowledgements
Acknowledgements: The authors thank all the volunteers and staff nurses who helped in completing the study. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: M.O.J. designed and implemented the research, conducted data analysis and wrote the paper. N.A.K., R.S. and I.A.N. were involved in designing and refining the study protocol, data analysis and reviewing the article. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Human Research Ethic Committee (The National University of Malaysia – Universiti Kebangsaan Malaysia) (code: NN-025-2015). Verbal consent was witnessed and formally recorded by the main researcher, M.O.J.












