Article contents
The structure and mechanical properties of Cu50Ni50 alloy nanofoams formed via polymeric templating
Published online by Cambridge University Press: 11 March 2020
Abstract
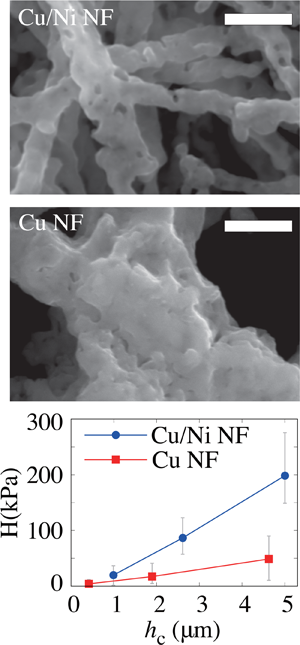
The authors demonstrate that multicomponent metallic alloy nanofoams can be synthesized by the polymeric templating method. The present approach enabled alloy compositions not accessible via commonly used dealloying or co-deposition methods. The authors report the synthesis of a Cu50Ni50 alloy nanofoam using electrospinning polymeric templating, which exhibits distinct polycrystallinity, process-driven segregation, and enhanced mechanical strength over pure Cu nanofoams. Transmission electron microscopy revealed microscopic grain formation and their variable compositions. The processing method is applicable to the synthesis of a wide range of multicomponent metal porous materials, creating new research opportunities for noble alloy foams not available through wet electrochemical routes.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 3
- Cited by




