1. Introduction
De-institutionalization has enabled women with schizophrenia to be more sexually active [Reference Solari, Dickson and Miller1] and the general fertility rate among them appears to have increased [Reference Vigod, Seeman, Ray, Anderson, Dennis and Grigoriadis2]. Since the 1960s, evidence has accumulated for an association between schizophrenia and various obstetric and postpartum complications [Reference Sobel3, Reference Rieder, Rosenthal, Wender and Blumenthal4]. The first meta-analysis [Reference Sacker, Done and Crow5] reported that births to women with schizophrenia incur an increased risk of pregnancy and birth complications, as well as low birthweight and poor neonatal conditions of the offspring.
In a Danish population-based study [Reference Bennedsen, Mortensen, Olesen and Henriksen6–Reference Bennedsen, Mortensen, Olesen and Henriksen8], women with schizophrenia were at increased risk of delivery interventions such as surgical delivery, vaginal assisted delivery, amniotomy, and pharmacological stimulation of labor. Their offspring were at increased risk of preterm delivery, being small for gestational age, low birth-weight, low Apgar scores, post-neonatal death, and congenital malformations as compared with newborns of unexposed mothers. A Swedish national population-based study [Reference Nilsson, Lichtenstein, Cnattingius, Murray and Hultman9] reported increased risks of preterm delivery, low birth-weight, being small for gestational age, stillbirth and infant death among newborns of women with schizophrenia. However, risk estimates were markedly reduced by controlling for maternal factors including: smoking during pregnancy, age, education, country of birth, pregnancy-induced hypertensive diseases, and parity. In an Australian population-based study [Reference Jablensky, Morgan, Zubrick, Bower and Yellachich10], women with schizophrenia showed an increased risk of placental abnormality, abruptio placentae, prolonged labor, precipitate delivery, cephalopelvic disproportion, antepartum hemorrhages and fetal distress. Risks of gestational age <37 weeks, birth-weight <2500 g, 5-min Apgar score <7, time to spontaneous respiration >2 min, intubation, drug side effects, and receiving narcotic antagonists were all elevated among babies of women with schizophrenia. However, after adjustment for maternal age, marital status, Aboriginal descent, parity, plurality, and sex of the offspring, the only complications that remained significant were abruptio placentae and naloxone administration. The incidence of birth defects including congenital malformations and chromosomal anomalies was only marginally elevated, but there was a significant increase in the incidence of defects of the cardiovascular system and minor physical anomalies. In a population-based study from Israel [Reference Hizkiyahu, Levy and Sheiner11], the need for induction of labor, augmentation of delivery, as well as low birth-weight (<2500 g) and congenital malformations of the offspring, were significantly increased among women with schizophrenia. No significant differences were observed in labor complications. According to a recent meta-study [Reference Matevosyan12], neonates to women with schizophrenia are profiled with intrauterine growth retardation, prematurity, low Apgar scores, and congenital defects. Additionally, the postpartum period typically involves psychotic relapse and parenting difficulties. However, after adjusting for maternal age, unhealthy behaviors, length of antipsychotic treatment, maternal-fetal attachment, as well as parity, maternal schizophrenia remains predictive of only prematurity and postpartum psychosis. In a Canadian population-based study [Reference Vigod, Kurdyak, Dennis, Gruneir, Newman and Seeman13], infants born to women with schizophrenia were at higher risk of prematurity, as well as of being either small or large for gestational age. These findings remained significant after adjustment with maternal pre-pregnancy medical comorbidity, age, socio-economic status and parity. Further, compared with age- and parity-matched controls, women with schizophrenia required significantly more intensive hospital resources, including operative delivery and admission to the intensive care unit.
Overall, population-based studies of deliveries of women with schizophrenia are still relatively scarce. Moreover, delivery is influenced by cultural and socio-economic conditions, as well as the provision and funding of health care services. Therefore, research findings may be context-specific, and the generalizability of findings between settings, countries, and time periods is thus uncertain. The aim of this Finnish register-based population study was to investigate obstetric and perinatal health outcomes in women with schizophrenia and their newborns. As post-hoc analyses, we explored associations between maternal smoking and unwanted perinatal health outcomes of the offspring, as well as time trends related to these outcomes.
2. Materials and methods
2.1 Participants
The study sample comprised a Finnish national population of women who were born between 1.1.1965–31.12.1980 and diagnosed with schizophrenia or schizoaffective disorder (=broadly-defined schizophrenia; here, schizophrenia) in specialized health-care at some point during the follow-up time ending 31.12.2013 (n = 5214). For each case, five controls were randomly selected from the Finnish Central Population Register, matched for age and place of birth, and who had not been diagnosed with schizophrenia, schizoaffective disorder or any other psychotic disorder by the end of the follow-up time. Other mental health disorders, such as depression or mood disorders, were allowed. The total final number of controls was 25,999 because for a few cases no control could be found due to the strict matching criteria.
2.2 Diagnoses of schizophrenia and schizoaffective disorder
The diagnoses were obtained from the Care Register for Health Care of the National Institute of Health and Welfare. In Finland, psychiatric classification according to the International Classification of Diseases – Eighth Revision (ICD-8) [Reference World Health Organization14] served in clinical practice between 1969 and 1986 (schizophrenia: 295.0-6, 295.8-9; schizoaffective psychosis: 295.7). This classification was later replaced by the Diagnostic and Statistical Manual of Mental Disorders −Third Revised Version (DSM-III-R) [Reference American Psychiatric Association15], used in clinical practice between 1987 and 1995. However, the diagnoses were converted to ICD-9 [Reference World Health Organization16] diagnoses, when, for example, reporting them to the Care Register for Health Care (schizophrenia: 295.0-6, 295.8-9; schizoaffective psychosis: 295.7). Since 1996, ICD-10 [Reference World Health Organization17] has been used in Finland (schizophrenia: F20; schizoaffective psychosis: F25). The onset of schizophrenia was defined as the day when the disorder was diagnosed and coded in specialized health care.
2.3 Follow-up
Women were followed from the onset of the disorder until the individual moved abroad, died, or follow-up ended on 31.12.2013. The information on death or emigration was gathered from the Finnish Central Population Register. The follow-up time of schizophrenic women was 14.0 (standard deviation [SD] 6.9) years, and, respectively, of controls 14.3 (SD 6.9) years (p =.001).
2.4 Information on obstetric and perinatal health outcomes
2.4.1 The Medical Birth Register
The Medical Birth Register has been maintained by the National Institute of Health and Welfare since 1987. It covers all delivery hospitals in Finland and includes data on live births and stillbirths of fetuses with a birth-weight of at least 500 g or a gestational age of at least 22 weeks, as well as data on the mothers. Individual data collection starts from the beginning of pregnancy and ends after one week from the delivery. Data quality studies indicate that most of the register content corresponds well or satisfactorily with hospital records [Reference Gissler, Teperi, Hemminki and Merilainen18]. In this study, all pregnancies leading to singleton births during the follow-up period were included, whereas multiple pregnancies were excluded. The following variables were collected: maternal age at birth, marital status at the end of pregnancy, smoking in the beginning of pregnancy, sex of the newborn, the number of deliveries, breech presentation (recorded since 1991), induction of labor (since 1991), epidural anesthesia (since 1991), use of forceps/vacuum, asphyxia (since 1991), delivery by cesarean section, delivery by elective cesarean section (since 1991), a perinatal death, gestational birth age (by fetal ultrasound examination at the first maternity care visit), premature birth (<37 weeks’ gestation), very premature birth (<28 weeks’ gestation), birth-weight, low birth-weight (<2500 g), very low birth-weight (<1500 g), low Apgar score at 1 min (<7), very low Apgar score at 1 min (<4), assisted ventilation (since 1991), resuscitation (since 1991), and neonatal monitoring (since 1991). Also, the following ICD-10 diagnoses (since 2004) were studied: precipitate labor (O62.3), prolonged delivery (O63), fetal distress (O68), labor and delivery complicated by umbilical cord complications (O69), rupture of perineum (O70), postpartum hemorrhage (O72), maternal distress (O75.0), puerperal sepsis (O85), other puerperal infections (O86), puerperal venous complications (O87), obstetric embolism (O88), puerperal psychosis (F53.1), and puerperal depression (F53.0).
2.4.2 The Finnish Register of Congenital Malformations
The Finnish Register of Congenital Malformations has been maintained by the National Institute of Health and Welfare since 1963. The register contains data on congenital chromosomal and structural anomalies detected in stillborn and live born infants and fetuses as well as terminations of pregnancy due to congenital anomaly. It collects data from hospitals, health-care professionals, and cytogenetic laboratories and draws data from other nationwide registers, and the completeness and validity of this register is considered to be good. We included only major congenital anomalies, multiple anomalies and syndromes, excluding minor anomalies according to the European Surveillance of Congenital Anomalies (EUROCAT) criteria [Reference EUROCAT Central Registry19].
2.5 Covariates
We used maternal age, marital status (single vs. married/cohabitation), smoking status (yes/no), and parity as covariates (model 1). Then, sex of the newborn was added to the model (model 2). The distribution of these variables is presented in Table 1.
Table 1 The distribution of background variables among women with schizophrenia and unexposed women
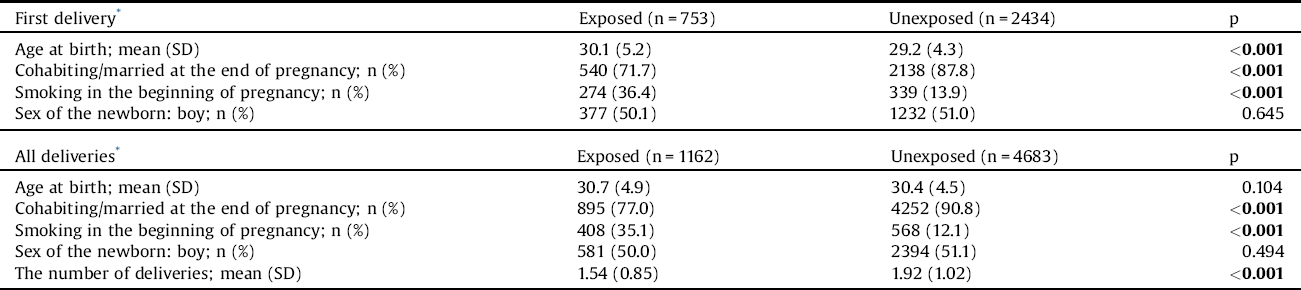
The Chi-square (x136) test and the independent samples t-test (age) were used to compare the groups.
The bold values are statistically significant.
* After being diagnosed with schizophrenia.
2.6 Data analysis
The analyses were performed in two ways; first, we included each woman’s first singleton pregnancy, which lead to a delivery after she was diagnosed with schizophrenia. Second, we included all of each woman’s singleton pregnancies that led to deliveries after she was diagnosed with schizophrenia. Chi square (x2) test, Fisher‘s exact test, the independent samples t-test and logistic regression analysis were used. With regard to all pregnancies, in order to account for the clustering of pregnancies within mothers, logistic regression analysis was performed with the generalized estimating equation (GEE) method. Analyses were performed both unadjusted and adjusted for the covariates described above. Variables with less than 10 affected women (in the schizophrenia group, or in the control group, or in both groups) were omitted since these models were considered unstable. Findings were considered significant when the two-tailed p < 0.05. The odds ratios (ORs) with 95% confidence intervals (CIs) are reported. Analyses were performed using SPSS 22.0 for Windows and SAS 9.3.
2.7 Ethical considerations
The Ethics Committee of Helsinki and Uusimaa Hospital District evaluated and approved the study plan. Permission to use the confidential register data in the study was granted by the National Institute for Health and Welfare and the Population Register Centre.
3. Results
The study focused on singleton pregnancies which led to a delivery after the index day (=the day when the disorder was coded in specialized health-care). This way, we identified 1162 singleton births among women with schizophrenia, of which 753 (64.8%) were the first births. We restricted the analysis of deliveries in controls to those that occurred after the index day of the case, which led to 4683 singleton births, of which 2434 (52.0%) were the first births.
3.1 Obstetric health outcomes
Regarding first deliveries, induction of labor and elective cesarean section were significantly more common among women with schizophrenia than controls (Table 2). With regard to all pregnancies, induction of labor, as well as deliveries by cesarean section and elective cesarean section were significantly more common among schizophrenic women than controls. Group comparisons related to the studied ICD-10 diagnoses revealed no statistically significant differences.
Table 2 Obstetric complications among women with schizophrenia and among unexposed women.
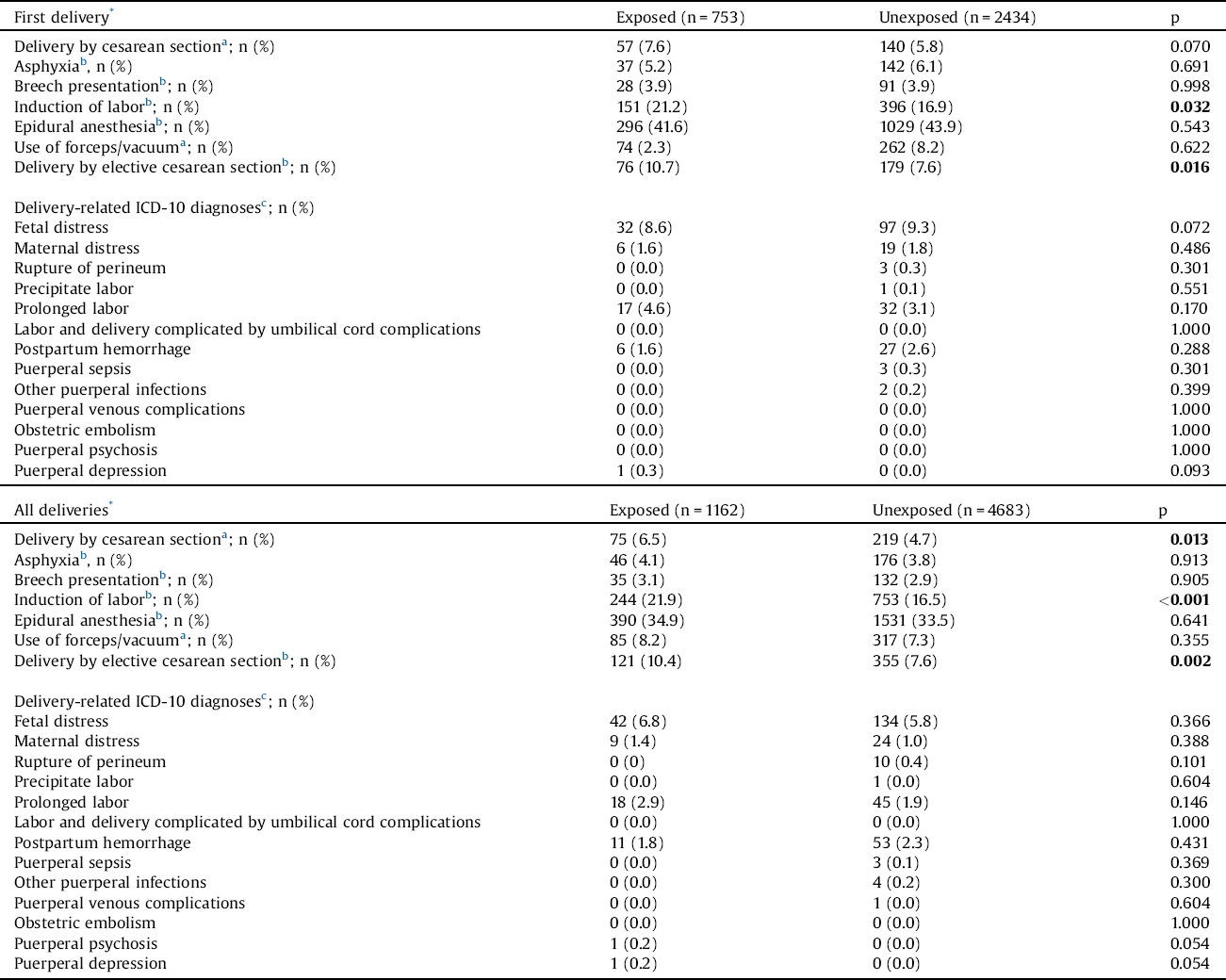
The Chi-square (x2) test, Fisher’s exact test and the independent samples t-test (gestational age, birthweight) were used to compare the groups.
The bold values are statistically significant.
* After being diagnosed with schizophrenia.
a Recorded since 1987.
b Recorded since 1991.
c Recorded since 2004.
3.2 Negative perinatal health outcomes of the offspring
Regarding first deliveries, gestational age and birth weight of offspring of women with schizophrenia were significantly lower than those of offspring of control women (Table 3). Also, premature birth, very premature birth, low birth-weight, very low birth-weight, resuscitation, and neonatal monitoring were significantly more common among offspring of schizophrenic women than among offspring of controls. With regard to all pregnancies, the same statistically significant differences were observed, but, in addition to this, low (<7) and very low (<4) Apgar score at 1 min, as well as assisted ventilation were significantly more common among offspring of women with schizophrenia than offspring of controls. Congenital anomalies were significantly more common among offspring of schizophrenic women than offspring of controls, for both first deliveries and all deliveries. With regard to different types of congenital anomalies, in first deliveries, syndromes were significantly more frequent among offspring of women with schizophrenia than offspring of controls. When all deliveries were taken into account, both syndromes and isolated anomalies were significantly more frequent among offspring of schizophrenic women than offspring of control women.
Table 3 Perinatal health outcomes among offspring of women with schizophrenia and offspring of unexposed women.
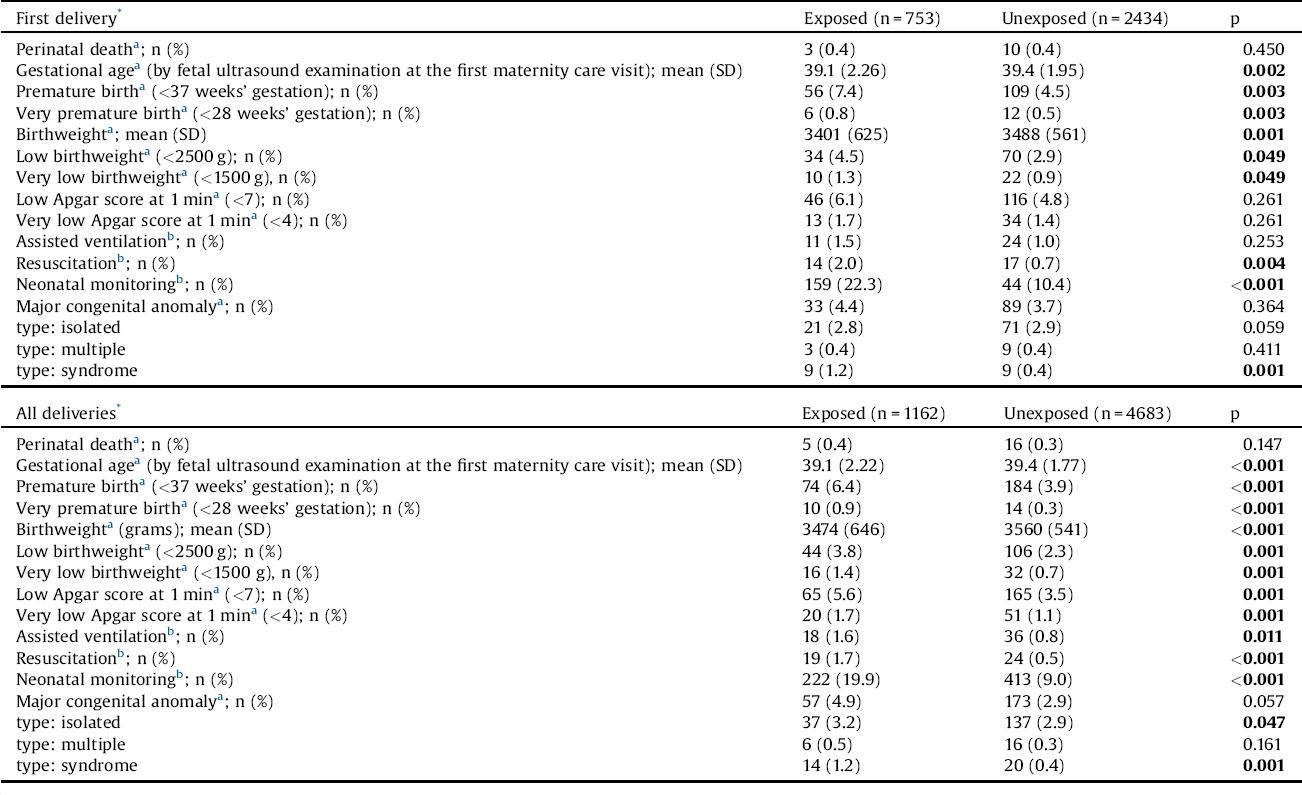
The Chi-square (x2) test, Fisher’s exact test and the independent samples t-test (gestational age, birthweight) were used to compare the groups.
The bold values are statistically significant.
* After being diagnosed with schizophrenia.
a Recorded since 1987.
b Recorded since 1991.
3.3 Associations between schizophrenia and obstetric health outcomes
Regarding first deliveries, the risk of induction of labor was 1.3-fold higher in women with schizophrenia (Table 4). With regard to all deliveries, the risk of induction of labor, delivery by cesarean section, and delivery by elective cesarean section was 1.4-fold higher. In adjusted models, all the above-mentioned differences remained statistically significant.
Table 4 The risk of obstetric complications in women with schizophrenia, with control women serving as a reference group.
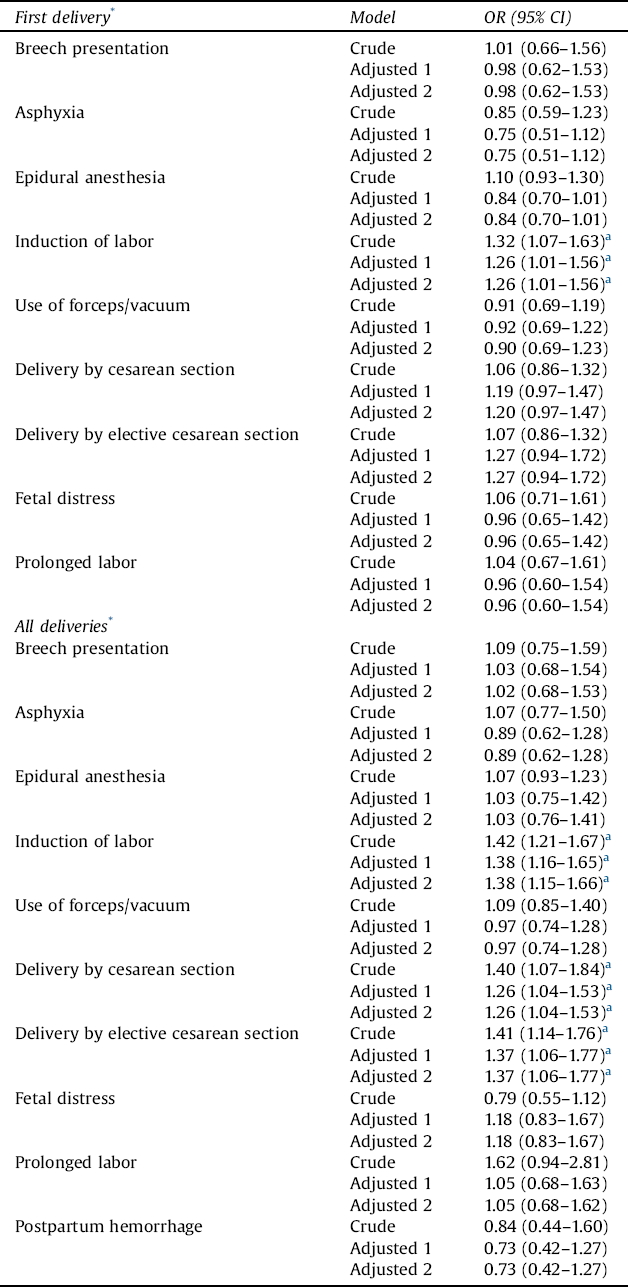
OR = odds ratio; CI = confidence interval
Results of logistic regression and generalized estimating equation (GEE) models are provided. Maternal age at birth, marital status (single vs. married or cohabitation), parity, and smoking status in the beginning of the pregnancy (yes/no) were used as covariates (Adjusted 1). Next, the previously mentioned variables and sex of the newborn were used as covariates (Adjusted 2).
* After being diagnosed with schizophrenia.
a Statistically significant finding.
3.4 Associations between maternal schizophrenia and negative perinatal health outcomes of the offspring
Regarding first deliveries, the risk of premature birth, low birthweight (<2500 g), low Apgar score at 1 min (<7), and congenital anomaly ranged from ORs of 1.4 to 1.7 among newborns of women with schizophrenia (Table 5). The risk of resuscitation was 2.7-fold and that of neonatal monitoring 2.5-fold higher. In adjusted models, only differences in the risks of premature birth and neonatal monitoring remained statistically significant. For all deliveries, the risk of premature birth, low birthweight (<2500 g), low Apgar score at 1 min (<7), and congenital anomaly ranged from ORs of 1.3 to 1.8. The risk of very low birth-weight (<1500 g) was 2.0-fold, assisted ventilation 2.2-fold, resuscitation 3.2-fold, and neonatal monitoring 2.4-fold higher. In adjusted models, only risk of premature birth, low Apgar score at 1 min (<7), resuscitation, and neonatal monitoring remained statistically significant.
Table 5 The risk of negative perinatal health outcome among offspring of women with schizophrenia, with control women serving as a reference group.
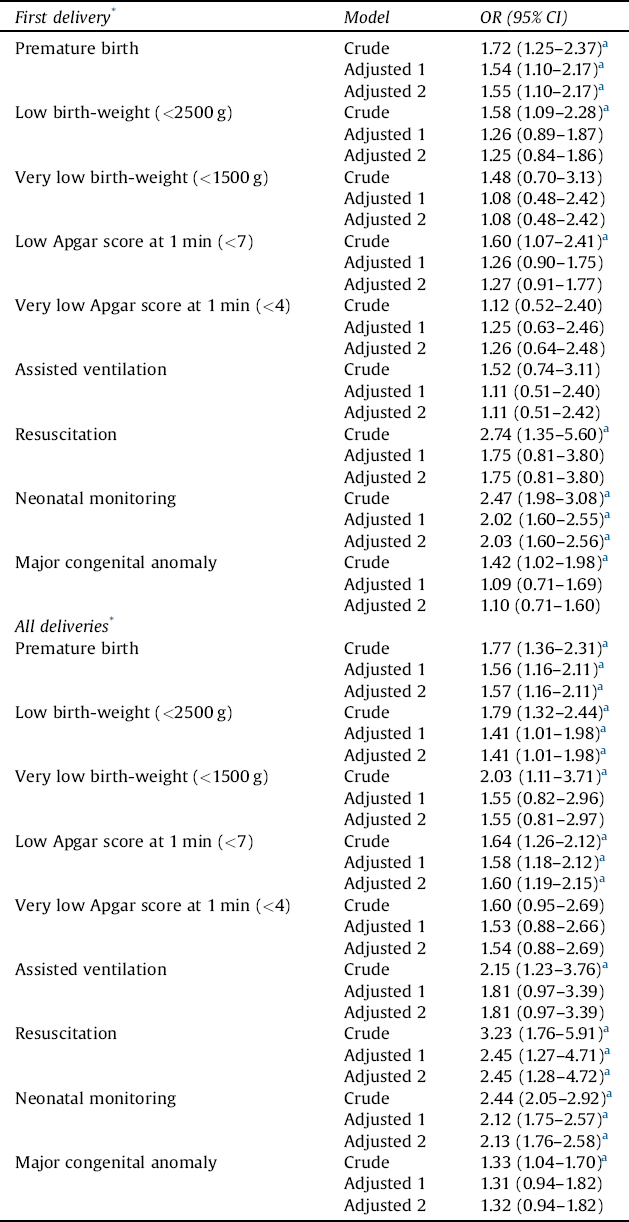
OR = odds ratio; CI = confidence interval.
Results of logistic regression and generalized estimating equation (GEE) models are provided. Maternal age at birth, marital status (single vs. married or cohabitation), parity, and smoking status in the beginning of the pregnancy (yes/no) were used as covariates (Adjusted 1). Next, the previously mentioned variables and sex of the newborn were used as covariates (Adjusted 2).
* After being diagnosed with schizophrenia.
a Statistically significant finding.
3.5 Associations between maternal smoking and negative perinatal health outcomes of the offspring
First, we focused on all women who smoked, with non-smoking women serving as the reference group. Birth year, maternal age, marital status, and parity were used as covariates. With regard to all deliveries, smoking was a significant risk factor for premature birth (OR 1.56, 95% CI 1.16–2.11), low birth-weight (OR 1.41, 95% CI 1.01–1.98), low Apgar score at 1 min (<7) (OR 1.58, 95% CI 1.18–2.13), and neonatal monitoring (OR 2.12, 95% CI 1.75–2.57). Then, we focused on women with schizophrenia who smoked, with non-smoking women with schizophrenia as the reference group. With regard to all deliveries, smoking was a significant risk factor for very low birth weight (OR 3.32, 95% CI 1.14–9.60) and neonatal monitoring (OR 1.50, 95% CI 1.10-2.05) after background adjustment.
3.6 Time trends
Before 2000, the risk of premature birth, very low birth weight (<1500 g), and resuscitation were significantly higher among newborns of women with schizophrenia than newborns of controls. However, during the years 2000 and 2013, this was no longer seen (Table 6). Before 2000, the risk of low Apgar score (1 min; <7) and the risk of congenital anomaly did not differ statistically between newborns of women with schizophrenia and control women, but, after 2000, these risks were significantly higher among newborns of schizophrenic women.
Table 6 The risk of offspring’s negative perinatal health outcome among women with schizophrenia in two different time periods, with control women serving as a reference group.
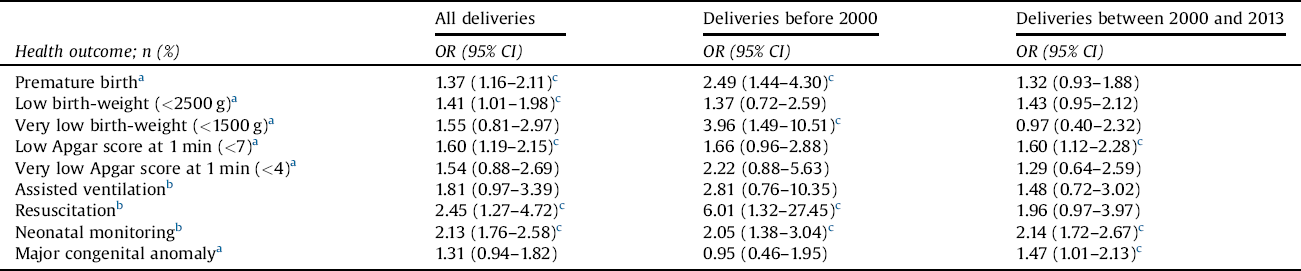
OR = odds ratio; CI = confidence interval.
Maternal age at birth, marital status (single vs. married or cohabitation), parity, smoking status in the beginning of the pregnancy (yes/no), and sex of the newborn were used as covariates.
a Recorded since 1987.
b Recorded since 1991.
c Statistically significant finding.
4. Discussion
This register-based, national population study comprised all singleton births among women who were born between 1965 and 1980 and diagnosed with schizophrenia until the end of 2013. We hypothesized schizophrenia to be associated with negative obstetric and perinatal health outcomes, but expected these associations to diminish after adjusting for age, smoking status, and marital status of the mother, as well as for parity and sex of the newborn. Indeed, after adjustment, only the risks of labor induction and delivery by cesarean section remained elevated. Delivery-related ICD-10 diagnoses were rare and, concordant with Hizkiyahu et al. [Reference Hizkiyahu, Levy and Sheiner11], no group differences were observed. In other words, obstetricians seem to use specific delivery methods more often in women with schizophrenia, but delivery-related complications are not increased.
The risk of premature birth, low Apgar score at 1 min (<7), resuscitation and neonatal monitoring were increased. The causes of unwanted perinatal health outcomes and the potential to prevent them remain unclear. Possible causative factors include abnormal fetal development due to a genetic predisposition, the effects of maternal illness and stress, comorbid problems such as sociodemographic disadvantage, poor nutrition and associated life style factors, poor attendance at antenatal care, or the effects of prescribed drugs [Reference Judd, Komiti, Sheehan, Newman, Cast and Everall20]. We could adjust for some of them, with little influence on our findings.
As a post-hoc analysis, we investigated associations between maternal smoking and unwanted perinatal health outcomes of the offspring. The stimulus for this emerged from the knowledge that the prevalence of smoking among women, as well as the prevalence of maternal smoking during pregnancy has declined substantially in the Nordic countries during the past twenty years, excluding Finland [Reference Ekblad, Gissler, Korkeila and Lehtonen21]. Approximately 16% of all Finnish women smoke daily and the steady prevalence of smoking among mothers-to-be is 15% [Reference Ekblad, Gissler, Korkeila and Lehtonen21]. Smoking turned out to be a risk factor for premature birth, low birth weight, low Apgar score at 1 min (<7), and neonatal monitoring. Thus, our finding agrees with the study by Jacobssen et al. [Reference Jacobsson, Gissler, Paavonen and Tapper22] indicating smoking to be one of the greatest risk factors for preterm delivery. Focusing on women with schizophrenia, smoking turned out to be a risk factor for very low birth weight and neonatal monitoring. The self-medication hypothesis [Reference Rüther, Bobes, De Hert, Svensson, Mann and Batra23], common genetic pathways [Reference Loukola, Wedenoja, Keskitalo-Vuokko, Broms, Korhonen and Ripatti24], as well as social factors like poverty and low education level [Reference Tidey and Miller25], have been proposed as an explanation for the strong relationship between nicotine dependency and schizophrenia. However, individuals with schizophrenia are known to be interested in and capable of smoking cessation [Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Schroeder26, Reference Gilbody, Peckham, Man, Mitchell, Li and Becque27]. Our findings underline the need for anti-smoking psychoeducation, as well as targeted smoking cessation interventions for women who plan pregnancy or find out that they are pregnant.
We explored time trends related to the risk of various negative perinatal health outcomes, because of the rising general standard of living, quality of medical care, and decreasing preterm delivery rates among Finnish women [Reference Jacobsson, Gissler, Paavonen and Tapper22]. We found that the risk of premature birth and the risk of very low birth weight of the newborn were both significantly higher among women with schizophrenia before 2000, but later this was no longer seen. Recently, Nguyen et al. [Reference Nguyen, Faulkner, Frayne, Allen, Hauck and Rock28] compared obstetric and neonatal outcomes of a sample of women with severe mental illness, who gave birth between December 2007 and April 2011, with those of the general population. They reported that, in contrast with earlier studies, the risk of preterm birth was not significantly greater compared with controls.
In Finland, approximately 4.8% of newborns have a major congenital anomaly, and this prevalence has remained constant [29]. An unexpected finding was that the risk of congenital anomaly was not significantly elevated among women with schizophrenia before 2000, but was almost 1.5-fold higher in 2000–2013. Given the more advanced currently available screening methods, in cases of recognized anomaly of the fetus, healthy women may induce abortion more often than women with schizophrenia. An important issue is whether our finding could be related to the growing use of atypical antipsychotics [Reference Hálfdánarson, Zoëga, Aagaard, Bernardo, Brandt and Fusté30]. In Finland, the prevalence of typical antipsychotics use was higher than the prevalence of atypical antipsychotics use in 2005, but the finding was the opposite in 2014 [Reference Hálfdánarson, Zoëga, Aagaard, Bernardo, Brandt and Fusté30]. More precisely, according to the national Drug Reimbursement Register (DRR), maintained by the Social Insurance Institution in Finland, the defined daily dose (DDD) of atypical antipsychotics per 1000 inhabitants/day was 3.5 in 1999 and 17.7 in 2013. In a recent study by Huybrechts et al. [Reference Huybrechts, Hernández-Díaz, Patorno, Desai, Mogun and Dejene31] with more than one million pregnant women, atypical antipsychotics showed no statistically significant risk for congenital malformations (relative risk [RR] 1.05, 95% CI 0.96–1.16). However, a small increased risk in malformations (RR 1.26, 95% CI 1.02–1.56) was found for risperidone. Depression often co-occurs with schizophrenia [Reference Siris32], and the use of antidepressants, especially selective serotonin reuptake inhibitors (SSRIs), has also increased substantially during the last decades [Reference McCarthy33]. This trend has also been observed in Finland [Reference Malm, Artama, Brown, Gissler, Gyllenberg and Hinkka-Yli-Salomäki34]. According to DRR, DDD of SSRIs per 1000 inhabitants/day was 21.4 in 1999 and 41.7 in 2013. Regarding pregnancy, 0.4% of all Finnish pregnant women used SSRI-medication in 1996, but, in 2010, this prevalence was already 3.8% [Reference Malm, Artama, Brown, Gissler, Gyllenberg and Hinkka-Yli-Salomäki34]. According to a cohort study with more than 600,000 offspring [Reference Malm, Artama, Gissler and Ritvanen35], the overall major congenital anomalies were no more common in SSRI-exposed offspring compared with unexposed ones (OR 1.08, 95% CI 0.96–1.22). However, fluoxetine was associated with an increased risk of isolated ventricular septal defects (OR 2.03, 95% CI 1.28–3.21), paroxetine of right ventricular outflow tract defects (OR 4.68, 95% CI 1.48–14.74), and citalopram of neural tube defects (OR 2.46, 95% CI 1.20–5.07). Overall, to understand more clearly how risks of various perinatal health outcomes vary in time and, especially, to find the clinical relevance from these findings, more time-trend analyses from different countries and cultures are obviously needed.
4.1 Strengths and limitations
The strengths of this study include our ability to investigate the Finnish national female population of patients with schizophrenia or schizoaffective disorder, the relatively long follow-up time, and the high quality of the Finnish health registers [Reference EUROCAT Central Registry19, Reference Aro, Koskinen and Keskimäki36]. Also, the diagnoses of psychotic disorders have been shown to be good [Reference Isohanni, Mäkikyrö, Moring, Räsänen, Hakko and Partanen37, Reference Pihlajamaa, Suvisaari, Henriksson, Heilä, Karjalainen and Koskela38]. However, some limitations need to be considered: first, we used an age- and place-of-birth-matched control group for comparison, but confounding factors such as socioeconomic status were not taken into account. This might have affected birth weight slightly. In Finland, most patients with schizophrenia are on disability pension [Reference Perälä, Saarni, Ostamo, Pirkola, Haukka and Härkänen39]. However, the municipal health-care services are funded by tax revenues and are available to all citizens regardless of their financial situation or employment. Second, we were limited to variables that were recorded in national registers described earlier. Unfortunately, we had no information about psychotropic or other medications prescribed to women nor the nature and amount of their alcohol or illicit substance use. Moreover, we did not have any paternal information even though this seems to be relevant as well [Reference Nilsson, Lichtenstein, Cnattingius, Murray and Hultman9]. Third, there may also be differences between individual clinicians and local customs within hospitals in the diagnosis and reporting of ICD-10 diagnoses related to childbirth. Fourth, we assumed that the onset of schizophrenia was the day when the disorder was diagnosed in specialized health-care, but, we had no information before this, for example on the onset and severity of psychiatric symptoms. Finally, considering the high number of outcomes, it must be acknowledged that some of the observed associations may have occurred by chance.
5. Conclusions
Schizophrenia associates with specific delivery methods but delivery complications are rare and their prevalence does not differ from that observed among community women. Maternal schizophrenia associates with some negative perinatal health outcomes of the offspring. Careful monitoring of mothers-to-be with schizophrenia, as well as intense co-operation between psychiatrists, gynecologists and obstetricians are recommended. Anti-smoking psychoeducation, as well as targeted smoking cessation interventions should be offered to schizophrenic women who plan pregnancy or find out that they are pregnant.
Funding sources and their roles
This study has been funded by the Helsinki and Uusimaa Hospital District. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributions
LS: study concept and design, collection and interpretation of data, and serving as a first author. EI: study concept and design, and critical revision of the manuscript for important intellectual content. MG: statistical analysis and interpretation of data, and drafting of the manuscript. JS: interpretation of data, and drafting of the manuscript. EH: interpretation of data, and drafting of the manuscript, NL: study concept and design, and interpretation of data, and critical revision of the manuscript for important intellectual content.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgement
The authors are most grateful to M. Grainger for her contribution to data management and computational issues.











Comments
No Comments have been published for this article.