Article contents
Portable solid rapid quantitative detection for Cu2+ ions: Tuning the detection range limits of fluorescent conducting polymer dots
Published online by Cambridge University Press: 27 March 2017
Abstract
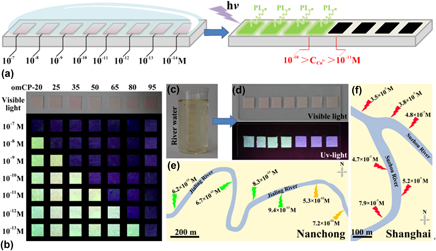
In this work, o-phenylenediamine-m-phenylenediamine copolymer dots (omCPs) with designed surface groups are synthesized and characterized. Here, we explored a simple, rapid semiquantitative detection system for Cu2+ with a wide detection range (5–7 orders of magnitude) based on the fluorescence in the solid state of omCPs and their tunable detection limits. The construction and application of the rapid semiquantitative detection system for Cu2+ are developed and demonstrated for the practical applications. What’s more, the detection limit can be modulated easily by adjusting the surface groups of these dots through the monomer dose control before the co-polymerization. Moreover, we demonstrated that this new technological approach is suitable for the semiquantitative determination of other ions pollutants (i.e., Na+, K+, Cu2+, Pb2+, Hg2+, and NO2 −) in environmental water.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
These authors contributed equally to this work.
Contributing Editor: Tao Xie
References
REFERENCES
- 1
- Cited by





