Key Points
Aging is associated with an increased incidence of cardiovascular diseases, including ischemic heart disease, heart failure, atrial fibrillation, hypertension, valvular heart disease, pulmonary hypertension, and peripheral vascular disease.
Progressive central aortic dilatation, increased thickness of the arterial wall, increased vascular stiffness, and altered nitric oxide–induced vasodilation occur with advancing age, leading to elevated mean arterial pressure and increased pulse pressure.
Elderly patients are more likely to present with non-ST-segment-elevation myocardial infarction (NSTEMI) as opposed to ST-segment-elevation myocardial infarction (STEMI), and frequently present with nonspecific complaints including weakness, syncope, and increasing confusion.
Overall 30-day mortality after coronary artery bypass grafting (CABG) in octogenarians is 6.8 versus 1.6 percent in the younger group.
The elderly comprise the majority of patients with heart failure with preserved ejection fraction (HFpEF).
The prevalence of moderate to severe aortic stenosis is estimated to be as high as 2.8 percent in patients older than 75 years of age, and complications associated with transcatheter aortic valve implantation (TAVI) appear to be greater in older patients.
Age is the most important risk factor for the development of atrial fibrillation (AF). New-onset postoperative AF (POAF) is a common problem reported in 15 to 40 percent of patients following CABG, 40 percent following surgical valve replacement, 50 to 60 percent following combined CAGB-valve procedures, 20 to 25 percent of patients after esophagectomy, and 20 percent of patients following lung transplant.
Meaningful outcome metrics in elderly patients who are admitted to the intensive care unit (ICU) and identifying those most likely to benefit from admission to the ICU still need to be delineated.
General Considerations
Practitioners of adult critical care medicine frequently encounter geriatric patients and by default are practitioners of geriatric critical care medicine. This may be even truer in the realm of cardiovascular medicine and cardiothoracic critical care. Aging is associated with an increased incidence of cardiovascular diseases, including ischemic heart disease, heart failure, atrial fibrillation, hypertension, valvular heart disease, pulmonary hypertension, and peripheral vascular disease [Reference Mozaffarian, Benjamin and Go1] (Figure 6.1). Significant technological advances have resulted in an increasing array of minimally invasive interventions that can now be used in patients who may have insufficient physiologic reserve to tolerate more extensive procedures. This in particular includes the elderly. Minimally invasive valve replacement, valve repair, implantable electrical devices, and an expanding armamentarium of endovascular approaches to aortic disease are just a few examples. These technological realities, in combination with the significant rise in the elderly populations, place a large responsibility on all practitioners delivering cardiovascular critical care to meet the needs of this highly variable, dynamic, and often challenging patient population.
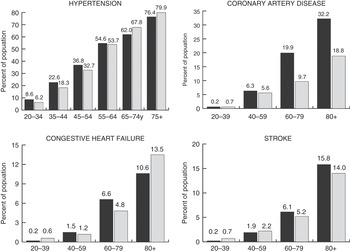
Figure 6.1 Prevalence of hypertension, coronary artery disease, congestive heart failure, and stroke with aging.
One may question what constitutes a working definition of geriatric cardiovascular critical care. In the simplest form, it is patient- and family-centered care that meets the needs of older adults. It includes a thorough and thoughtful consideration of age, comorbidities, and the available evidence to manage critical illness. Age alone should not be the deciding factor in determining therapeutic approach. Chronologic age and physiologic reserve can be incongruent. In many cases the presence of geriatric syndromes including frailty or dementia may influence outcome but do not necessarily correlate with patient age. Implicit in these considerations is a need to define the goals of care or intervention, including the expected outcome, patient values, and an understanding that aging is associated with an increase in the frequency and severity of iatrogenic complications and risk of harm from any treatment. Goals of care may differ widely depending on patient values and may change within a given period of care. Historically, outcome metrics have been largely preoccupied with mortality statistics, but an increasing emphasis is now been given to functional outcomes. In general, the therapeutic goal of most elderly patients should be to improve or maintain functional independence or alleviate pain. The complexity of medical decision making is increased by the potential effect of comorbidities and geriatric syndromes on the outcome of a specific therapy or intervention. In many situations, few data are available on which to base this estimate. Furthermore, these considerations take place in an environment of increasing cost containment and emphasis on value-based care. In considering value-based care, an understanding of expected outcomes is very important because the relationship of quality or outcome to cost is the defining principle of value-based care. Unfortunately, meaningful functional outcome data for cardiovascular therapies in critically ill elderly are sparse. However, some recent studies have sought to address these deficiencies. This chapter provides an overview of common cardiovascular problems that elderly patients encounter leading to admission to the ICU and their evidence-based management.
Cardiovascular Aging
Primary changes in cardiac function occur with advancing age. Morphologic changes include decreased myocyte number, increased collagen-to-elastin ratio, thickening of the left ventricular wall, and decreases in both conduction fiber density and the number of sinus node cells [Reference Priebe1a]. Adrenergic activity and receptors also change with age [Reference Brodde and Michel2]. These changes affect function, leading to decreased contractility, increased myocardial stiffness, increased ventricular filling pressures, and decreased β-adrenergic sensitivity.
Aging is associated with stiffening of the vasculature, or arterial aging, which leads to important secondary changes in the heart and other end organs, including the brain and kidney. Arterial aging is accelerated in the presence of cardiovascular comorbidities, including atherosclerosis, hypertension, diabetes, tobacco abuse, and obesity [Reference O’Rourke, Adji, Namasivayam and Mok2a–Reference Steppan, Barodka, Berkowitz and Nyhan5].
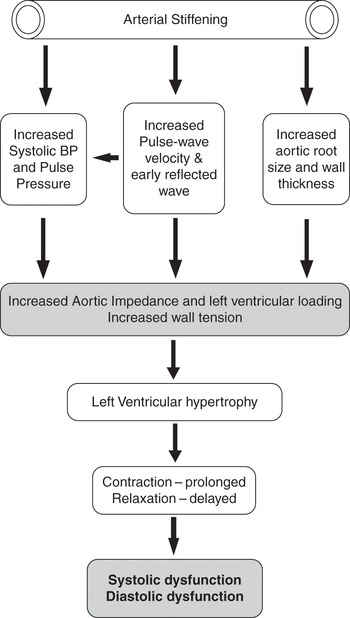
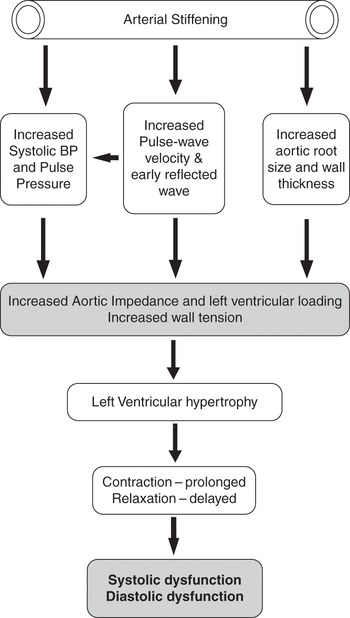
Increased vascular stiffness leads to increased velocity of conduction of pulse waves down the vascular tree, resulting in earlier reflection of pulse waves from the periphery such that reflected pulse waves reach the heart during the latter phases of ejection leading to increased cardiac load [Reference Steppan, Barodka, Berkowitz and Nyhan5]. This effect is evident on the arterial pressure tracing as late systolic peaking [Reference O’Rourke, Kelly, Avolio, O’Rourke, Kelly and Avolio6]. Increased left ventricular afterload leads to left ventricular wall thickening, hypertrophy, and impaired diastolic filling [Reference Frenneaux and Williams7] (Figure 6.2). Cardiac contraction is prolonged to compensate for decreased ventricular compliance and increased afterload, resulting in decreased early diastolic filling time. With these changes, the atrial contribution to late ventricular filling becomes more important and explains in part the clinically observed preload sensitivity and hemodynamic compromise often associated with failure to maintain sinus rhythm in elderly patients.
In the young, there is pulse pressure amplification as pulse waves travel down the vascular tree. This is observed as an increase in systolic pressure of 10 to 15 mmHg between the central aorta and the periphery with a slight decrease in diastolic and mean pressures. This a function of the cushioning effect of a compliant vasculature. With aging, this is lost resulting in an augmentation of central aortic pressure and increased impedance to left ventricular (LV) ejection [Reference Barodka, Joshi, Berkowitz, Hogue and Nyhan8]. Progressive central aortic dilatation, increased thickness of the arterial wall, increased vascular stiffness, and altered nitric oxide–induced vasodilation occur with advancing age, leading to elevated mean arterial pressure and increased pulse pressure [Reference Brodde and Michel2–Reference Steppan, Barodka, Berkowitz and Nyhan5] (Figure 6.2).
With aging there is a decrease in response to β-receptor stimulation and an increase in sympathetic nervous system activity [Reference Rooke9]. This occurs as a result of both decreased receptor affinity and alterations in signal transduction [Reference Rooke10]. With any physiologic stress, increased flow demands are placed on the heart. Attenuated β-receptor response in the elderly during stress is associated with decreased chronotropic and inotropic responses. In turn, the increased peripheral flow demand is met primarily by preload reserve, making the heart more susceptible to cardiac failure [Reference Mozaffarian, Benjamin and Go1]. While β-receptor responsiveness is decreased, sympathetic nervous system activity increases with aging and may be another mechanism contributing to increased systemic vascular resistance [Reference Mozaffarian, Benjamin and Go1]. Clinically, these autonomic changes lead to the heightened sensitivity of elderly patients to sympatholytic medications, with a greater likelihood of perioperative hemodynamic lability and a compromised ability to meet the metabolic demands of surgery.
Coronary Heart Disease: Acute Coronary Syndromes
Coronary heart disease (CHD) is a common problem in the elderly, with patients older than 65 and 75 years of age accounting for over half and over one-third of all patients, respectively, with a primary diagnosis of CHD at hospital discharge [Reference Goldberg, McCormick and Gurwitz11,Reference Roger, Jacobsen and Weston12]. This same group experiences a considerably higher mortality rate, comprising approximately 82 percent of all deaths from CHD [Reference Ezekowitz and Kaul13]. Modern cardiovascular care following acute myocardial infarction is associated with increased survival for the elderly, but survivors experience an increased incidence of heart failure [Reference Ezekowitz, Kaul and Bakal14]. The incidence of CHD and the burden of acute coronary syndromes (ACS) are expected to increase with projected growth in the population of older adults, increasing overall life expectancy, and a greater number of patients living longer with CHD due to improved therapies.
The elderly are underrepresented in previously published clinical trials of therapies for ischemic heart disease. In early studies, patients older than age 75 comprised only 2 percent of the patients enrolled. Over the past 20 years, this number has increased but falls short of reflecting the prevalence of CHD among the elderly [Reference Saunderson, Brogan and Simms15]. While limited randomized, controlled trial data are available to offer clear treatment recommendations, current management is informed by existing clinical trial data, observational studies, and clinical guidelines. It has been well described, however, that treatment as recommended by clinical consensus guidelines is frequently underutilized in the elderly. Clinician concerns regarding patient safety are generally cited for this deficiency [Reference McCune, McKavanagh and Menown16,Reference Zaman, Stirling and Shepstone17]. In 2007, the American Heart Association published a scientific statement on acute coronary syndromes in the elderly that includes discussions of considerations in ST-segment-elevation and non-ST-segment-elevation myocardial infarction [Reference Alexander, Newby and Armstrong18,Reference Alexander, Newby and Cannon19] Recent updates to clinical guidelines for the management of ACS have recommended similar management for all, without age-specific differences. Nevertheless, it is important to appreciate that the elderly present with a different clinical profile with ACS. Older patients more frequently experience baseline functional limitations, compensated heart failure, past history of ischemic heart disease, and chronic kidney disease [Reference Mehta, Rathore and Radford20]. The elderly are more likely to have an unusual presentation with nonspecific complaints, including weakness, syncope, or increasing confusion [Reference Bayer, Chadha, Farag and Pathy24]. This is important because atypical presentation can lead to delay in diagnosis and treatment. Evaluation may be further confounded by the presence of left bundle-branch block on electrocardiogram or elevated baseline levels of cardiac biomarkers in the aged [Reference Dai, Busby-Whitehead and Alexander21,Reference Normann, Mueller and Biener22]. Furthermore, elderly patients are more likely to present with non-ST-segment-elevation myocardial infarction (NSTEMI) as opposed to ST-segment-elevation myocardial infarction (STEMI) [Reference Roger, Weston and Gerber23]. It has been well documented that delays in treatment and a greater frequency of triage decisions result in less intensive treatment in older patients presenting with ACS [Reference Alexander, Newby and Cannon19]. It is unclear whether this is the result of atypical presentation, frequency of concurrent illnesses, or clinical bias.
Increasing age is associated with an increasing incidence of heart failure as a complication of acute myocardial infarction with an incidence as high as 65 percent in patients older than 85 years of age [Reference Mehta, Rathore and Radford20]. As mentioned previously, multiple studies have suggested that more aggressive therapies are frequently withheld in the elderly [Reference Mehta, Rathore and Radford20,Reference Paul, O’Gara and Mahjoub25,Reference Stone, Thompson and Anderson26]. While the elderly experience more heart failure following ACS than do their younger counterparts, including cardiogenic shock, bleeding, and in-hospital mortality, data suggest that with appropriate therapy a lower in-hospital mortality rate can be achieved [Reference Alexander, Roe and Chen27,Reference Avezum, Makdisse and Spencer28]. Considerations for elders presenting with STEMI are similar to those for all patients with regard to route and timing of reperfusion interventions. Fibrinolysis is of known benefit in the elderly. Elderly patients have a greater absolute mortality benefit than patients younger than age 55. However, this mortality benefit comes at a greater risk of bleeding and stroke, with stroke rates as high as 2.9 percent in patients older than age 85 having been reported [Reference Gore, Granger and Simoons29]. Overall outcomes with regard to stroke, recurrent myocardial infarction, and mortality tend to be better with percutaneous coronary intervention [Reference Ezekowitz and Kaul13,Reference Alexander, Newby and Armstrong18]. For this reason, fibrinolytic therapy generally should be considered only in clinical situations where there is confirmed STEMI presenting within 12 hours of symptom onset, no contraindications to treatments, and an expected time greater than 120 minutes to first-device activation for percutaneous coronary intervention (PCI) [Reference Dai, Busby-Whitehead and Alexander21].
Initial NSTEMI management decisions include consideration for early invasive versus conservative therapy. Data support an early invasive approach for elderly patients meeting criteria because it has been associated with increased survival and decreased reinfarction rates [Reference Bach, Cannon and Weintraub30]. An increasing number of patients older than age 75 and greater are presenting for open coronary artery revascularization [Reference Seco, Edelman and Forrest31]. For carefully selected patients with multivessel disease, surgery remains an option. One meta-analysis comparing PCI with CABG reported no significant difference in all-cause mortality at 30 days and 12 and 22 months. There was a higher rate of stroke in the CAGB patients but a greater need for repeat revascularization in the PCI group [Reference Alam, Virani and Shahzad32]. Off-pump CABG (OPCABG) has been proposed as a technique to limit complications associated with cardiopulmonary bypass and the associated aortic cannulation and cross-clamping. A trial of OPCABG specifically enrolling patients older than age 75 years failed to demonstrate benefit for OPCABG regarding death, myocardial infarction, stroke, need for renal replacement therapy, or need for repeat revascularization [Reference Diegeler, Borgermann and Kappert33]. Consistent with other published OPCABG trials, the OPCABG group required fewer blood transfusions. Emergent open revascularization remains associated with increased mortality risk. As may be anticipated, elderly patients experience longer lengths of hospital stay following management of ACS or CABG [Reference Alabas, Allan, McLenachan, Feltbower and Gale34,Reference Chee, Filion, Haider, Pilote and Eisenberg35]. Frailty also contributes to greater length of stay and higher rates of discharge to institutional care facilities [Reference Ekerstad, Swahn and Janzon36,Reference Lee, Buth, Martin, Yip and Hirsch37]. Overall 30-day mortality after CABG in octogenarians is 6.8 percent versus 1.6 percent in the younger group [Reference Sen, Niemann and Roth37a].
Heart Failure
Heart failure (HF) is common in the elderly, with the prevalence increasing exponentially with age. It is estimated that HF affects 6 to 10 percent of people older than 65 years of age. It is the leading cause of hospitalization in the elderly, with the majority of hospital inpatients between 70 and 75 years of age. Approximately 60 percent of elderly patients presenting with HF are women. A number of conditions are frequently present in patients presenting with HF, including atrial fibrillation, valvular heart disease, and dilated cardiomyopathy. Hospitalization for an episode of decompensated HF is associated with a high rate of readmission to the hospital and a 1-year mortality rate of nearly 30 percent. Patients may require ICU admission for acute decompensated heart failure (ADHF) or have chronic heart failure as a significant comorbidity in the setting of admission for another primary diagnosis. Clinical presentation of acute HF syndromes includes ADHF, acute pulmonary edema with normal blood pressure, acute pulmonary edema with hypertension, cardiogenic shock, high-output heart failure, and isolated right ventricular failure [Reference Gheorghiade, Zannad and Sopko38]. Heart failure can occur in the setting of reduced or preserved ejection fraction, although the elderly comprise the majority of patients with HF with preserved ejection fraction (HFpEF). The elderly may be less aggressively treated than younger patients with HF, and there is evidence of less use of diagnostic modalities and guideline-recommended therapeutic interventions [Reference Katsanos, Bistola and Parissis39]. The clinical presentation of HF is usually associated with congestion of the pulmonary and systemic vasculature and may include evidence of end-organ hypoperfusion. Therapeutic interventions may be directed at several targets to address these problems. Diuretics and vasodilators remain mainstays of therapy for HF. Heart failure with reduced ejection fraction (HFrEF) can be distinguished from diastolic dysfunction or HFpEF with echocardiography. Diastolic dysfunction is associated with decreased LV compliance and increased intracavitary pressures, which, in turn, result in increased pulmonary venous pressure. This can lead to frank HF and may be further exacerbated by conditions frequently encountered in ICU patients, such as volume overload, hypertension, and atrial fibrillation [Reference Vignon40].
Patients with HF complicated by hypoxemia may require mechanical ventilation. Noninvasive positive-pressure ventilation (NIPPV) has demonstrated efficacy in the management of HF [Reference Vital, Ladeira and Atallah41]. Elderly patients appear to benefit from this therapy as well, but patients should be carefully selected because contraindications to NIPPV, including altered mental status and an inability to clear secretions and protect the airway, may be present [Reference L’Her, Duquesne and Girou42,Reference Ozsancak Ugurlu, Sidhom and Khodabandeh43].
Long-term mechanical circulatory support (MCS) may be considered for subsets of elderly patients with end-stage HF. Ideally, selection criteria should include careful assessment of geriatric syndromes that may limit success or be incongruent with patient-centered values [Reference Vitale, Chandekar, Rodgers, Pagani and Malani44]. Clear guidelines for use of long-term MCS in elderly patients are not available but should be based on the demonstration of improved and cost-effective outcomes compared with standard medical therapy [Reference Hiesinger, Boyd and Woo45]. Studies have suggested safe implantation of continuous-flow devices in elderly patients, including those older than 70 years of age [Reference Kilic, Sultan and Yuh46]. Age, however, is associated with a greater likelihood of discharge to care facilities and is not unexpectedly a predictor of increased mortality in the elderly population [Reference Kilic, Sultan and Yuh46,Reference Atluri, Goldstone and Kobrin47].
Patients with HF may experience ICU admission in the setting of noncardiac surgery, and up to 25 percent of these patients experience an acute exacerbation of HF in the perioperative period [Reference Smit-Fun and Buhre48]. In this population, HF is strongly associated with increased perioperative mortality [Reference van Diepen, Bakal, McAlister and Ezekowitz49]. An increasing body of evidence suggests that routine use of inotropic medication is associated with harm [Reference Nielsen, Hansen and Johnsen50,Reference Nielsen and Algotsson51]. A recent meta-analysis, however, provides a contrary viewpoint [Reference Belletti, Castro and Silvetti52]. In light of the available evidence, it is prudent to consider limiting use of inotropic agents to patients with clinical evidence of low-cardiac-output states and impaired perfusion.
Valvular Heart Disease
Valvular heart disease, especially aortic stenosis (AS), is a frequently encountered problem in the elderly. The prevalence of moderate to severe aortic stenosis is estimated to be as high as 2.8 percent in patients over 75 years of age. As a comparison, the prevalence in the 18- to 45-year-old age group is 0.2 percent [Reference Nkomo, Gardin and Skelton53]. The only treatment that has been demonstrated to improve quality of life and increase survival is replacement of the valve. Historically, up to one-third of elderly patients with severe AS were not considered surgical candidates due to advanced age, LV dysfunction, or presence of significant comorbidities [Reference Lindman, Alexander, O’Gara and Afilalo54]. Mortality rate after surgical aortic valve replacement (AVR) in patients younger than 70 years of age is 1.3 percent, which increases to 5 percent in patients 80 to 95 years of age, with an increase to 10 percent in those older than 90 years of age.
Transcatheter aortic valve implantation (TAVI) techniques greatly expand treatment options for patients deemed to be at high surgical risk for open approaches. Overall, the available outcome data suggest symptomatic relief and survival advantage following TAVI in most, but not all, patients. Increasingly, emphasis has been placed on the attempt to determine which patients are most likely to benefit from intervention. This is a complex decision that does not currently have a clear answer because much remains to be learned concerning long-term outcomes in the elderly.
Technical aspects of TAVI include the type of valve implanted and the approach. The two most commonly used valves are the Edwards SAPIEN and the Core Valve, but technology is rapidly evolving, with newer generations of devices employing design features to decrease known complications. The Edwards SAPIEN is a balloon-expandable valve, and the Core Valve is self-expanding. Either valve can be placed via a retrograde approach through the femoral or axillary artery. For patients with significant peripheral vascular disease, transapical placement can be performed via a minithoracotomy. While early studies suggested no significant difference in mortality between transpical and transvascular approaches, it now appears that there is a survival advantage of transfemoral over transapical approaches [Reference Biancari, Rosato and D’Errigo55–Reference Johansson, Nozohoor and Kimblad57]. During the valve deployment, rapid ventricular pacing to heart rates as high as 200 beats per minute is used to minimize cardiac ejection and cardiac motion so as to facilitate deployment of the valve. These periods may result in hemodynamic compromise, including the risk of myocardial ischemia with delayed recovery, especially affecting patients with poor ventricular function. This, in turn, may require inotropic or vasopressor support with obvious implications for postoperative management.
Several studies have examined outcomes of TAVI compared with open surgical AVR (SAVR). Most studies are observational in nature, but randomized, controlled trials have been conducted. The published data suggest better in-hospital recovery for TAVI with similar short- and long-term mortality. Much of the literature has been informed by various publications resulting from the Placement of AoRtic TraNscathetER Valves (PARTNER) trial. PARTNER trial was a multicenter, randomized trial comparing TAVI with SAVR and medical therapy in high-risk patients, with groups consisting of patients estimated to be at high surgical risk (group A) and a group not judged to be surgical candidates (group B). These publications demonstrate noninferiority for TAVI versus SAVR in high-risk surgical patients with evidence of improved functional status and quality of life in some subgroups [Reference Reynolds, Magnuson and Wang58–Reference Makkar, Fontana and Jilaihawi62]. PARTNER trial data demonstrated similar rates of stroke, myocardial infarction, acute kidney injury, endocarditis, and permanent pacemaker placement at 1 and 2 years following TAVI or SAVR. TAVI was associated with a higher rate of vascular injury, and SAVR was associated with higher rates of major bleeding complications [Reference Kodali, Williams and Smith63]. Other studies have reported higher rates of vascular injury, permanent atrioventricular (AV) block, and residual aortic valve regurgitation with TAVI [Reference D’Errigo, Barbanti and Ranucci64].
Complications of TAVI appear to be greater in older patients. The most common complication after TAVI is vascular injury, including arterial dissection, perforation, and acute thrombosis. Patients undergoing TAVI are at risk for stroke. The incidence of stroke following TAVI has been reported to be from 2.5 percent to as high as 10 percent. Most strokes are ischemic in origin and are believed to be due to showering of emboli from the aorta during valve positioning and deployment. The majority of cerebral embolic events may be silent, as suggested by studies reporting new MRI findings in as many as 64 percent of patients following TAVI, with few patients manifesting any clinical signs of cerebral impairment [Reference Ghanem, Kocurek and Sinning65]. The high-risk group from the PARTNER trial demonstrated a trend toward a greater incidence of stroke in TAVI versus SAVR that did not reach statistical significance [Reference Smith, Leon and Mack61]. TAVI is associated with a risk of conduction system injury that may require permanent pacemaker placement. The Core Valve appeared to have a greater risk of AV conduction problems compared with the Edwards SAPIEN valve. This may be explained by the self-expanding design of the valve structure, which includes a longer frame. Patients with underlying right bundle-branch block appear to be especially at risk to require permanent pacemaker placement owing to the relative risk of injury to the left bundle-branch pathway during valve deployment. Acute kidney injury (AKI) is observed following TAVI. In several studies that have evaluated risk factors for AKI following TAVI, patient age, quantity of intravenous contrast material delivered, and preexisting renal disease were not predictive of the development of AKI. Paravalvular leak can lead to aortic regurgitation (AR) and appears to be more common in TAVI than in SAVR. It is more likely to occur with self-expanding valves compared with balloon dilated devices. It is believed that central AR is the result of higher-grade paravalvular regurgitation after TAVI. Mild central AR appears to be well tolerated, but early severe AR is associated with increased mortality after TAVI [Reference Tamburino, Capodanno and Ramondo66,Reference Athappan, Patvardhan and Tuzcu67]. In such cases, repeat valve replacement via valve-in-valve TAVI techniques can be considered. Valve malposition can occur and is usually noted at the time of valve deployment. Various complications can result, including embolization of the valve, blockage of the coronary ostia, paravalvular leak, interference with movement of the mitral valve leaflets, and dysrhythmias [Reference Huffmyer, Tashjian, Raphael and Jaeger68].
Postoperative considerations are largely the result of the physiologic consequences of valve replacement, underlying LV function, and the anticipated potential complications mentioned previously. Patients with severe AS often have impaired diastolic function related to long-standing increased in wall tension from the stenotic valve. Early after valve replacement, diastolic function has been observed to worsen in some cases [Reference Huffmyer, Tashjian, Raphael and Jaeger68]. Hemodynamic goals include avoidance of hypertension and maintenance of perfusion. Care should be taken to avoid pharmacologic agents that promote AV nodal blockade in patients with conduction system complications.
Much as TAVI has created treatment options for high-risk patients with severe AS, technological advances leading to techniques for transcatheter mitral valve repair have offered therapeutic considerations for high-risk patients with mitral regurgitation (MR) [Reference Taramasso, Candreva and Pozzoli69]. Degenerative MR in the elderly is a common problem, with significant MR demonstrated in over 10 percent of hospitalized patients older than 75 years of age [Reference Taramasso, Gaemperli and Maisano70]. Many of the same treatment dilemmas face elderly patients with MR as those with AS. Specifically, it is imperative to determine those most likely to benefit from treatment and align the available therapies with the goals of patients and their families. Realistic goals often address improvements in quality of life and functional status as opposed to prolonging survival [Reference Mirabel, Iung and Baron71]. As in other cardiac surgical populations, age is associated with risk in mitral valve surgery, with mortality rates reported to be 4.1 percent for those younger than age 50 and 17.0 percent for those older than age 80. Current data suggest that mitral valve repair is preferred to mitral valve replacement in the elderly. It is associated with decreased surgical mortality, lower risk of hemolysis and infection, avoidance of long-term anticoagulation, and improved long-term outcomes. However, open valve repair or replacement remains associated with poor functional recovery in the elderly [Reference Mehta, Eagle and Coombs72]. The randomized multicenter EVEREST II trial compared percutaneous mitral valve repair to open surgery in low-risk patients with MR, concluding that percutaneous repair was not inferior to open surgery [Reference Mauri, Foster and Glower73]. A high-risk cohort from the EVEREST II trial defined as estimated perioperative mortality of 12 percent or greater experienced a 30-day mortality of 6.7 percent, with survivors demonstrating improvement in New York Heart Association (NYHA) functional class and decreased hospital admission rates for heart failure [Reference Glower, Kar and Trento74].
Dysrhythmia
Through a variety of mechanisms, aging is associated with an increase in the prevalence of cardiac rhythm disturbances. Elderly patients may be admitted to the ICU for monitoring and management of primary dysrhythmic events or experience rhythm disturbances in the context of cardiac surgery, noncardiac surgery, or other acute critical illness. As is the case with many conditions in the aging population, presentation of disease may be atypical, and there is a risk of greater sensitivity to both therapeutic effects and adverse drug effects of medications frequently used to treat the underlying problem. Atrial fibrillation and bradydysrhythmias are particularly common in the elderly, and an increasing number of older patients have been considered for implantation of cardioverter-defibrillators for both primary and secondary management of malignant ventricular rhythms.
Bradydysrhythmias can be classified generally as abnormalities related to impulse generation or impulse conduction. Frequent underlying problems involve decreased automaticity of the sinus node or delay or blocked conduction in the sinus node or AV node or His-Purkinje system [Reference Kumar, Kusumoto and Goldschlager75]. Aging has been associated with an increase in the prevalence of sinus node dysfunction, AV nodal block, and bundle-branch block. A wide variety of pathologic processes can contribute to conduction system disease, including age-related degeneration, ischemia, infection, infiltrative diseases, and trauma, including post–cardiac surgery effects. Secondary factors may also contribute to dysrhythmias and are frequently encountered in the ICU. These are electrolyte derangements, temperature imbalance, disorders of gas exchange, hypothyroidism, and adverse pharmacologic effects of medications. Treatment for symptomatic bradycardia frequently requires permanent pacemaker placement. Clinical trials suggest advantages for dual-chamber pacing over ventricular pacing alone and include a lower incidence of heart failure, reduction in the incidence of pacemaker syndrome, and a decreased incidence of the development of atrial fibrillation [Reference Lamas, Lee and Sweeney76]. The observed advantages have been attributed to preservation of AV synchrony. However, long-term isolated right ventricular pacing may lead to an increased incidence of heart failure due to functional left bundle-branch block leading to ventricular dysynchrony [Reference Kumar, Kusumoto and Goldschlager75].
Excluding a history of atrial fibrillation (AF), age is the most important risk factor for the development of AF [Reference Hakim and Shen77]. Elderly patients may suffer from paroxysmal or persistent AF. New-onset AF is also frequently encountered in the ICU and is a well-described complication following cardiothoracic surgery, high-risk noncardiac surgery, trauma, or critical illness, including sepsis. The elderly are especially predisposed to developing AF due to structural and electrical changes observed with aging. Infiltrative processes and fibrosis lead to a reduction in atrial myocytes and nodal pacemaker cells, resulting in alterations in excitable tissue and conduction. These changes, along with age-related dilatation and remodeling of the left atrium and alterations in calcium conductance and potassium currents, create a milieu favoring AF [Reference Hakim and Shen77]. Other risks factors commonly found in elderly patients that have been associated with AF include hypertension, diabetes mellitus, ischemic heart disease, valvular heart disease, heart failure, obesity, and chronic obstructive pulmonary disease (COPD).
New-onset postoperative AF (POAF) is a common problem reported in 15 to 40 percent of patients following CABG, 40 percent following surgical valve replacement, and 50 to 60 percent following combined CAGB-valve procedures [Reference Hogue, Creswell, Gutterman and Fleisher78]. Atrial fibrillation is also observed following noncardiac thoracic surgery, with a reported incidence of 12 to 30 percent in patients having pulmonary resection, 20 to 25 percent in patients undergoing esophagectomy, up to 50 percent of patients requiring extrapleural pneumonectomy, and 20 percent of patients following lung transplant [Reference Vaporciyan, Correa and Rice79–Reference Mason, Marsh and Alster81]. A number of conditions exist following cardiothoracic procedures that strongly favor precipitation of AF. Operative trauma, myocardial ischemia, oxidative stress, ischemia-reperfusion injury, inflammation, increased atrial pressure, volume overload, alterations in autonomic tone, exogenous catecholamines, other inotropic agents, electrolyte disturbances, disordered acid-base homeostasis, altered gas exchange, and pain may interact with age-related changes in cardiac structure and conduction leading to slower conduction through normal pathways and shortened refractoriness that predispose to AF [Reference Echahidi, Pibarot, O’Hara and Mathieu82]. When AF occurs in the ICU setting, the severity of symptoms will influence the mode of therapy. Immediate synchronized electrical cardioversion is indicated for patients with acute instability including AF associated with hypotension, chest pain, shortness of breath, altered mental status, and acute congestive heart failure. Cardioversion enjoys high success rates, but when AF is not terminated, it is important to distinguish between conversion failure and early reinitiation of AF [Reference Crawford and Oral83]. In conversion failure, cardioversion may be attempted at a higher energy level or after administration of an antiarrhythmic medication. If early reinitiation is the problem, repeated attempts at cardioversion are less likely to be effective until secondary factors have been addressed [Reference Rho84]. If AF is well tolerated hemodynamically, a decision must be made regarding rate versus rhythm control and timing and duration of anticoagulation. Institutional and individual practitioner preferences often affect this decision. A rate-control strategy appears to be safe and effective in elderly patients with normal LV function. Rhythm control is often an attractive therapeutic goal for patients who remain symptomatic with adequate rate control. Other potential advantages associated with rhythm control include decreased time to cardioversion, prolonged maintenance of normal sinus rhythm, and decreased hospital length of stay, but recent data do not support a long-term advantage for rhythm control in post–cardiac surgical patients [Reference Gillinov, Bagiella and Moskowitz85]. Choice of antiarrhythmic agents is often dictated by the patient’s underlying ventricular function because many agents exhibit negative inotropic effects and a greater risk of pro-arrhythmia in those with structural heart disease. For patients with permanent AF and poor tolerance of medical management, AF ablation or AV nodal ablation and permanent pacemaker placement may be considered [Reference Hakim and Shen77].
Use of implantable cardioverter-defibrillators (ICDs) is common in the elderly, with an estimated 40 percent or more of ICDs and cardiac resynchronization therapy (CRT) devices implanted in those older than 70 years of age. The obvious questions regarding life expectancy and survival benefit are largely unanswered. The incidence of sudden cardiac death as a percentage of all-cause mortality decreases with age. Device implantation appears to be well tolerated [Reference Barra, Providencia, Paiva, Heck and Agarwal86].
Vascular Disease
An increasing array of options for the treatment of aortic and peripheral arterial disease has led to a rapid increase in the use of minimally invasive approaches to aortic aneurysm repair and treatment of carotid artery stenosis and critical limb ischemia. These interventions are attractive because they promise less physiologic stress, greater tolerance, and fewer periprocedure complications in patients with limited organ reserve. These procedures, however, have the potential to introduce new procedural risks and may not consistently produce better long-term outcomes congruent with the goals of patients because long-term data on functional outcomes are inconsistent or limited.
Endovascular repairs for both abdominal aortic aneurysms and thoracic aneurysms, as well as a variety of hybrid procedures for complex aortic disease, are now commonly performed. Endovascular approaches have been associated with a reduction in early morbidity and mortality compared with open approaches. Patients may be admitted to the ICU for hemodynamic and neurovascular monitoring, hemodynamic management, and management of lumbar drains for the prevention of spinal cord ischemia or for the management of associated complications. Associated complications may include AKI, postoperative bleeding, mesenteric ischemia, spinal cord ischemia, myocardial ischemia or infarction, distal embolization, AF, respiratory failure, and stroke. Technical issues with endovascular grafts may lead to different types of endoleaks. Age is not an absolute contraindication to endovascular aortic repair, and several reports suggest efficacy in octogenarians [Reference Lange, Leurs, Buth and Myhre87,Reference Henebiens, Vahl and Koelemay88]. Nonagenarians have also been reported to tolerate endovascular aortic repair (EVAR) well but may not have the same mortality benefit compared with their younger counterparts [Reference Wigley, Shantikumar and Hameed89]. This overall lack of benefit may be related to increased all-cause mortality after EVAR that compares with the high expected mortality of nonagenarians as a whole. Mortality data fail to consider quality of life, and there is evidence that physical performance remains impaired at 12 months in both open and endovascular aortic repair. These aspects of expected outcome should be considered in the treatment of the oldest old. While EVAR is associated with better early survival, the risk of late rupture appears greater than with open surgical repair [Reference Schermerhorn, Buck and O’Malley90].
Carotid disease is more prevalent in the elderly and often is present in patients with significant comorbidities. Following surgical procedures to treat symptomatic carotid stenosis, patients may be admitted to the ICU for hemodynamic monitoring and management and frequent serial neurologic assessments. Recent advancements in endovascular therapy have made carotid stenting an alternative approach to traditional carotid endarterectomy (CEA). Perioperative complications such as myocardial infarction, surgical site bleeding, and cranial nerve palsy occur less frequently with stenting. However, the risk of stroke is increased with stenting versus CEA [Reference Blackshear, Cutlip and Roubin91–Reference Ouyang, Jiang, Yu, Zhang and Huang93]. A Cochrane Systematic Review reported the risk of procedural stroke or death with stenting to be 8.2 percent versus 5.0 percent for CEA (odds ratio [OR] 1.72, 95 percent confidence interval [CI] 1.29–2.31) [Reference Bonati, Lyrer, Ederle, Featherstone and Brown94]. A pooled analysis of several trials reporting an 8.9 percent incidence of stroke or death with stenting compared with 5.8 percent with CEA (relative risk [RR] 1.53, 95 percent CI 1.20–1.95). This difference, however, was strongly influenced by age, with an increased incidence of stroke and death only demonstrated in patients older than 70 years of age [Reference Bonati and Dobson95]. Both treatments are comparable in the long-term prevention of recurrent stroke [Reference Brott, Howard and Roubin96].
Treatment of critical limb ischemia in the elderly is controversial. Patients often have severe comorbidities, poor functional status at baseline, and poor functional outcome [Reference Duggan, Woodson, Scott, Ortega and Menzoian97]. Newer percutaneous approaches, including angioplasty, stenting, and atherectomy, may be better tolerated than traditional lower-extremity bypass, but outcome data are lacking [Reference Siracuse, Menard and Eslami98]. Vogel et al. reported that endovascular procedures may not lead to better functional outcome as assessed by activities of daily living (ADLs) with critical limb ischemia compared with open approaches [Reference Vogel, Petroski and Kruse99]. These results underscore the complexity of aligning treatment options with realistic goals where consistent outcome data are limited.
Other Considerations for Cardiovascular Critical Care of the Elderly
As noted previously, high-quality data to guide critical care of elderly cardiovascular patients are limited. Extrapolation of data from general ICU populations, with consideration of specific implications of the physiologic changes of aging affecting organ function, pharmacokinetics, and pharmacodynamics, along with baseline comorbidities, often drive the treatment approach in clinical practice. Other issues of concern for elderly patients in the ICU include delirium, postoperative cognitive dysfunction, and functional disability. The effects of the interaction of age, premorbid functional status, and geriatric syndromes on cardiovascular care are incompletely understood.
Delirium has an incidence of approximately 30 percent in cardiac surgical and cardiology patients [Reference Cropsey, Kennedy, Han and Pandharipande100,Reference Rudolph, Jones and Levkoff101]. Several risk factors have been identified in cardiac surgery patients, including a prior history of transient ischemic attack (TIA) or stroke, lower baseline Mini-Mental Status Examination scores, higher scores on screening for geriatric depression, and low serum albumin and low cardiac output states, including patients treated with intra-aortic balloon counterpulsation [Reference Rudolph, Jones and Levkoff101,Reference Norkiene, Ringaitiene and Misiuriene102]. As in other ICU populations, it is prudent to screen for delirium in the cardiovascular ICU and employ nonpharmacologic strategies in an effort to decrease the incidence, including providing appropriate lighting condition to encourage diurnal variation, frequent reorientations, ensuring that patients are in possession of their visual or hearing aids, optimizing sleep hygiene, minimizing medical devices as possible, and avoiding the use of physical restraints. Early physical therapy and early mobilization may decrease duration of delirium [Reference Collinsworth, Priest, Campbell, Vasilevskis and Masica103]. Medications known to exacerbate delirium should be avoided. Antipsychotic medications, including haloperidol, and atypical agents, including quetiapine and risperidone, have been used in the pharmacologic management of delirium. Data on efficacy are mixed. One trial in cardiac surgical patients suggested therapeutic efficacy for risperidone in decreasing the incidence of delirium [Reference Hakim, Othman and Naoum104].
Postoperative cognitive decline has been widely reported in patients following cardiac surgery. Published studies have used various methods of testing and timing of test administration, making interpretation challenging [Reference Bartels, McDonagh, Newman and Mathew105]. A number of potential contributing etiologic factors have been explored, including cardiopulmonary bypass associated inflammation, cerebral ischemia, hypoxemia, and intraoperative blood pressure management. The data are conflicting, and it appears that patient factors, including preexisting cognitive dysfunction and educational status, may be more significant risk factors [Reference Selnes, Grega and Bailey106,Reference Selnes, Gottesman and Grega107]. Duration of delirium has been correlated with cognitive decline but may be a marker for decreased cerebral reserve [Reference Pandharipande, Girard and Jackson108].
While a standardized definition has not been universally accepted, the concept of frailty may prove to be important in surgical decision making for cardiac surgical patients [Reference Morley, Vellas and van Kan109]. Frailty assessment has the potential to identify patients who are more likely to sustain lasting disability following surgical intervention [Reference Graham and Brown110]. In several studies of cardiac surgical patients, frailty as assessed by a variety of measures has been associated with increased perioperative morbidity and mortality [Reference Lee, Buth, Martin, Yip and Hirsch111–Reference Sepehri, Beggs and Hassan113]. Inclusion of frailty metrics in frequently employed surgical risk assessment tools appears to improve performance [Reference Afilalo, Mottillo and Eisenberg114]. The implications of frailty on specific ICU-related therapies and interventions is unknown, but it is prudent to address evaluation of cognitive status, mobility, pain control, and nutrition. Use of geriatric consultation or inclusion of geriatric specialists as part of a multidisciplinary team in the ICU may be useful in defining goals of care that optimize patient-centered outcomes [Reference Mohanty, Rosenthal and Russell115].
Areas of Uncertainty
Significant and rapid advances in technology have produced a growing array of therapies for cardiovascular diseases that have a variety of perceived advantages in the treatment of elderly patients. Minimally invasive modalities offer a lesser degree of physiologic stress that is frequently better tolerated by patients with decreased end-organ reserve. As with any progressive field, the available options may evolve faster than outcome data on which to base their implementation. Identifying patients most likely to benefit from aggressive intensive care and novel interventions is not an easy task. In patients with significant comorbidities, it may be difficult to clinically differentiate between the extent to which the cardiac disease is contributing to symptoms and functional limitation versus other coexisting conditions. Geriatric-specific syndromes and associated conditions including frailty, disability, impaired mobility, cognitive dysfunction, poor nutrition, polypharmacy, fall risk, mood disorders, and social isolation may influence prognosis in the elderly and may be more important determinants of outcome than traditional risk scoring systems [Reference Bell, Orr and Dodson116]. In addition, individual therapy must carefully consider what outcomes are acceptable to patients and their families with an appreciation of the potential for goals of care to change over the course of an episode of care. In pursuing the best outcomes for elders with cardiovascular disease, much is yet to be learned. Rich et al. have proposed a research agenda for issues specific to the management of cardiovascular disorders [Reference Rich, Chyun and Skolnick117]. Future research should include a patient-centered approach to delineating meaningful outcome metrics, defining and eliciting the impact of geriatric syndromes and commonly encountered comorbidities on outcome, and defining the patients most likely to benefit from admission to intensive care.