26% of English adults are classified as obese and a further 35% are overweight(Reference Baker1), reflecting the fact that the UK has the 6th highest rate of obesity globally(Reference Baker1). Levels of obesity are rising alarmingly despite 75% of adults reportedly attempting to lose weight(Reference Baker1). The ‘3500 kcal rule’ or 500 kcal/day restriction, is used frequently in clinical practice and should result in a weight-loss of 1 pound/week(Reference Thomas2) though is consistently shown to overestimate weight-loss(Reference Thomas2). This can be explained by physiological adaptations – adaptive thermogenesis – designed to protect our body's energy stores(Reference Miller4) that manifest as a depression in resting metabolic rate(Reference Redman and Ravussin3). These dynamic changes are unaccounted for in current weight-loss strategies. We therefore aimed to propose an alternative mathematical approach to weight-loss that accounts for the contribution of adaptive thermogenesis, as well as diet- induced thermogenesis and body-composition.
The initial model used baseline values of weight, energy intake and energy expenditure. Values for resting metabolic rate were estimated using predictor equations(Reference Miller4) and values for body-composition were obtained from relevant studies(Reference Gallagher and Delegge5, Reference Ghoch6). A basic model was defined and produced as an ordinal differential equation illustrating a static model of weight-loss. The model was refined through addition of an accurate formulation of adaptive thermogenesis. The new function was solved numerically with appropriate parameters. The final model included an accurate formulation of fat-free mass. The model was validated by comparison to three independent weight-loss studies varying the degree of caloric restriction(Reference Vink7), macronutrient content(Reference Veum8) and physical-activity level(Reference Redman9).
The most accurate results were observed in the study varying physical-activity level, where our model predicted weight-loss to 100% accuracy in both the caloric-restriction and the caloric-restriction-plus exercise groups. A high degree of accuracy was also observed in the study varying degree of caloric restriction, with predicted weight-loss deviating only by 1 and 2 kg from observed weight-loss in the low-calorie-diet and very-low-calorie-diet groups, respectively. The largest margin of error was observed in the macronutrient-manipulation study, with differences of 5–7 kg observed between predicted and actual weight-loss in the high-fat and high-carbohydrate groups, respectively (Table 1).
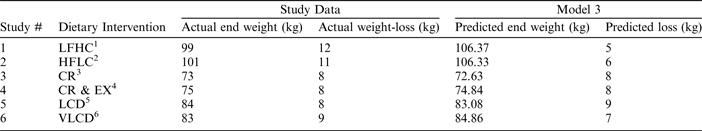
1 Low-fat high-carbohydrate
2 High-fat low-carbohydrate
3 Caloric restriction
4 Energy expended by exercise
5 Low-calorie diet
6 Very low-calorie diet
Our model provides further evidence challenging the 3500 kcal rule by illustrating the physiological influence of adaptive thermogenesis, diet-induced thermogenesis and body-composition on weight-loss. It predicts weight-loss achieved through caloric restriction and exercise to a high level of accuracy, however further refinement is needed to account for the impact of macronutrient composition on weight-loss. Minimal input is required to generate accurate results making our model highly applicable in a clinical environment.





