Article contents
Synthetic biology for the development of bio-based binders for greener construction materials
Published online by Cambridge University Press: 24 April 2019
Abstract
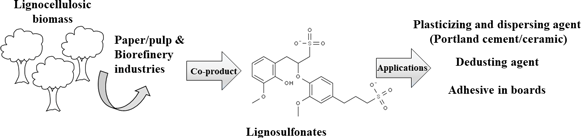
The development of more sustainable construction materials is a crucial step toward the reduction of CO2 emissions to mitigate climate change issues and minimize environmental impacts of the associated industries. Therefore, there is a growing demand for bio-based binders which are not only safer toward human and environmental health but also facilitate cleaner disposal of the construction materials and enable their compostability. Here, we summarize the most relevant bio-based polymers and molecules with applications in the construction sector. Due to the biologic nature of these materials, the existing biotechnologic processes, including synthetic biology, for their development and production have been evaluated.
- Type
- Synthetic Biology Prospectives
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 2
- Cited by




