Summary Box
Head and neck 2
Anatomy assessment 2
Abnormal skull 3
‘Butterfly sign’ is absent 6
Intracranial translucency is absent 7
Intracranial translucency is enlarged 8
Nasal bone is absent 9
Micrognathia is suspected 10
Cleft lip and palate are suspected 11
Large nuchal translucency 12
Jugular lymphatic sacs 14
Cystic hygroma 15
Heart 16
Anatomy assessment 16
Abnormal four-chamber view 19
Abnormal three-vessel view 21
Aberrant right subclavian artery (ARSA) 22
Chest 23
Anatomy assessment 23
Mediastinal shift 24
Pleural effusion 26
Spine 26
Anatomy assessment 26
Meningomyelocele, meningocele 27
Abdomen 28
Anatomy assessment 28
Exomphalos 28
Gastroschisis 30
Ectopia cordis, pentalogy of Cantrell or body stalk anomaly? 30
Genitourinary system 31
Anatomy assessment 31
Bladder is large (megacystis) 32
Skeletal system 33
Anatomy assessment 33
Limb abnormalities 34
Short femur 39
Hydrops 40
Placenta and umbilical cord 40
Anatomy assessment 40
Single umbilical artery 41
Umbilical cysts 41
Head and Neck
Anatomy Assessment
Head
– Cranial bones, midline falx
– Ventricles filled with choroid plexus – ‘butterfly’ sign (Fig. 1.1)
– Intracranial translucency/posterior fossa
Face
Neck
– Normal appearance
– Nuchal translucency (Fig. 1.4)
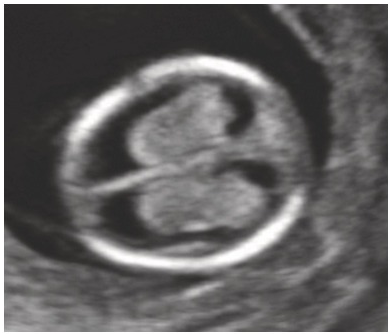
Fig. 1.1 Transverse view of the head. The appearance of the normal choroid plexus is called the ‘butterfly sign’. Note that there is no space between the choroid plexus and the walls of the lateral ventricle.
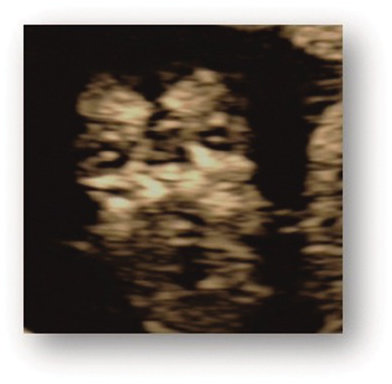
Fig. 1.2 Posterior coronal view of a normal face with large anterior fontanelle. The orbits are seen with the white dot indicating the lenses.
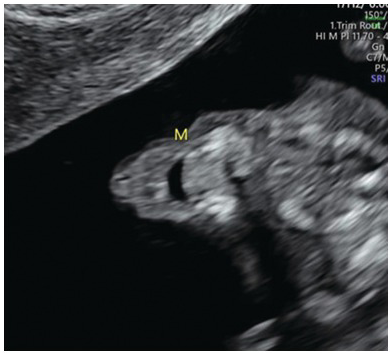
Fig. 1.3 Anterior coronal view of a normal fetal face with open mouth and intact lips.
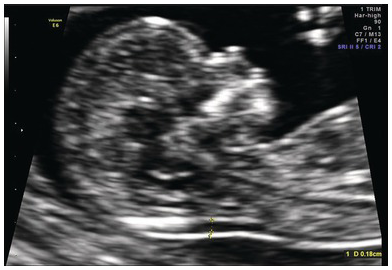
Fig. 1.4 Normal fetal profile with visible nasal bone and normal nuchal translucency (1.8 mm).
Abnormal Skull
Acrania–Exencephaly–Anencephaly Sequence (Fig. 1.5)
Definitions and Characteristics
Amorphous brain mass – exencephaly (Fig. 1.6)
Commonly associated with neural tube abnormalities (craniorachischisis, spina bifida, iniencephaly) and facial, cardiac, renal and gastrointestinal malformations
Ultrasound Assessment
Use transvaginal scan to exclude the presence of amniotic band sequence
Investigations
Karyotyping may reveal an aneuploidy which would alter the risk for future pregnancy
Counselling and Management
Offer termination of pregnancy
Offer palliative care and discuss organ donation programme, if available
Recurrence is ~3–4%
Amniotic band sequence is sporadic with no increased risk for future pregnancies
Advise folic acid supplementation (5 mg/day) in all future pregnancies
Skull Defects
Definitions and Characteristics
Defects are most commonly occipital (85%) and may contain:
The clear distinction may not be accurate in the first trimester as contents of a defect may vary at later gestations
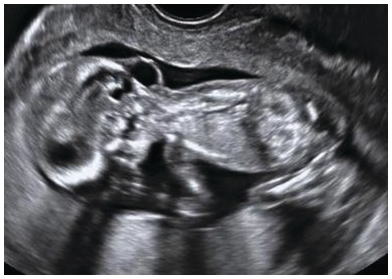
Fig. 1.7 Cystic swelling behind the occipital bone communicating with the posterior fossa through a small calvarial defect suggestive of occipital meningocele. This has to be differentiated from cystic hygroma or lymph cysts, wherein there will be no communication with intracranial structures.
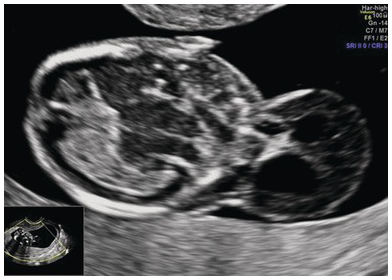
Fig. 1.8 Transverse view of an occipital encephalocele. Note the presence of the brain matter, calvarial defect and altered intracranial anatomy.
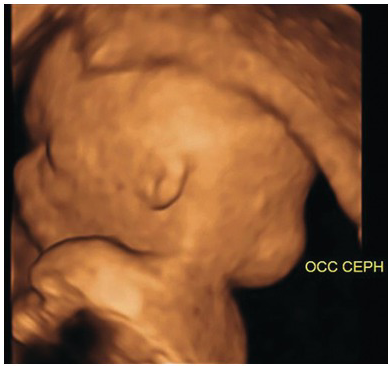
Fig. 1.9 3D view of an occipital encephalocele.
Ultrasound Assessment
Investigations
Counselling and Management
‘Butterfly Sign’ Is Absent
Definition and Characteristics
Ultrasound Assessment
Look for signs of alobar holoprosencephaly (single ventricle, fused thalami, absent falx) (Fig. 1.10)
Less severe forms of holoprosencephaly are unlikely to be detectable in the first trimester
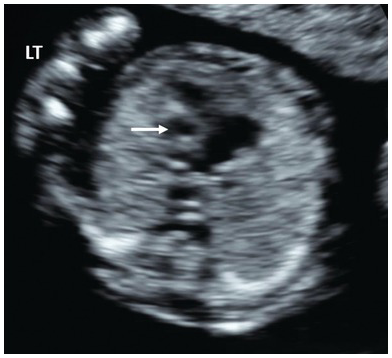
Early ventriculomegaly presents as choroid plexus filling less than half of the ventricular space and not touching the medial and lateral ventricular walls (Fig. 1.11)
Check for facial clefts and proboscis (a trunk-like nose)
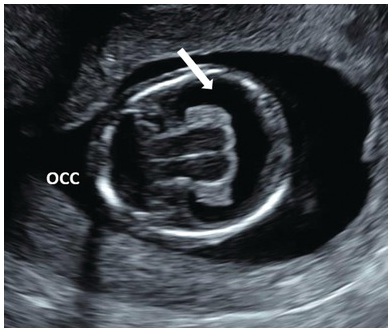
Fig. 1.10 Note the single ventricle (arrow) with absent midline falx and fused thalamus. The ‘butterfly’ sign is absent. Occ, Occiput.
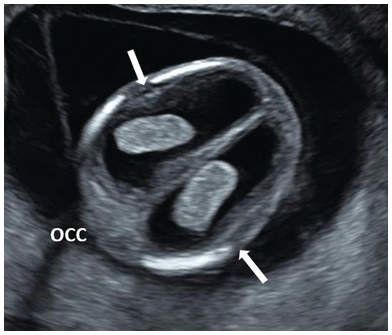
Fig. 1.11 Dilated lateral ventricles (arrows) are suspected in the first trimester when there is a visible space between the choroid plexus and the lateral wall of the lateral ventricles. Occ, Occiput.
Investigations
Offer invasive testing
Counselling
Alobar holoprosencephaly is usually lethal in early childhood; up to 80% of fetuses will have abnormal karyotype, mainly trisomy 13 (60%), but also trisomy 18, triploidy and deletions
Ventriculomegaly in the first trimester is often associated with chromosomal and posterior fossa abnormalities, but can also be ‘isolated’ or transient, with good long-term prognosis
Intracranial Translucency Is Absent
Definition and Characteristics
Intracranial translucency (IT) is the fourth ventricle which is seen in the mid-sagittal view of the head
The IT is parallel to the nuchal translucency and appears between two echogenic borders – brainstem anteriorly and choroid plexus of fourth ventricle posteriorly (Fig. 1.12)
Immediately below the fourth ventricle is a small translucent area representing the cerebromedullary cistern, which later forms the cisterna magna
The IT increases from 1.5 mm at a CRL of 45 mm to 2.5 mm at a CRL of 84 mm
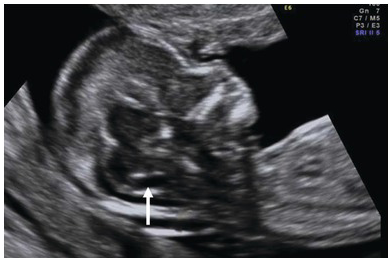
Fig. 1.12 Sagittal plane of the face with normal IT (arrow) which represents the fourth ventricle which lies between the brain stem (above) and the cisterna magna (below).
Ultrasound Assessment
Absent or small IT in sagittal section (Fig. 1.13)
In axial sections the choroid plexus appears to ‘fill’ the hemisphere and is often referred to as the ‘dried up brain’ (Fig. 1.14), or the ‘crash’ sign in the transverse plane (Fig. 1.15)
Look for spinal defect – it may not be obvious even when using the transvaginal approach
Absence of lemon and banana signs is not reassuring enough – these signs are not typically present before 14 weeks
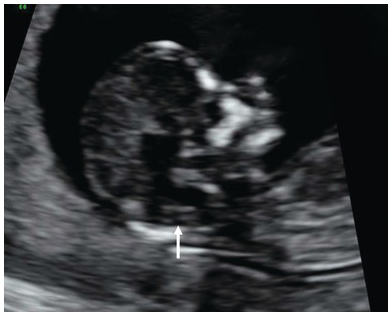
Fig. 1.13 Sagittal plane of the face in a fetus with an open neural tube defect. IT is absent due to the descent of the medulla oblongata through the foramen magnum and compression of the fourth ventricle (arrow).
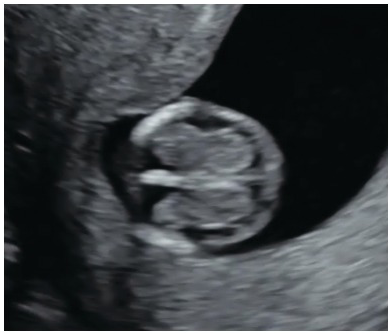
Fig. 1.14 Transverse section of the head in a fetus with spina bifida. The choroid plexus appears to fill up the entire hemisphere. This is referred to as the ‘dried up brain’.
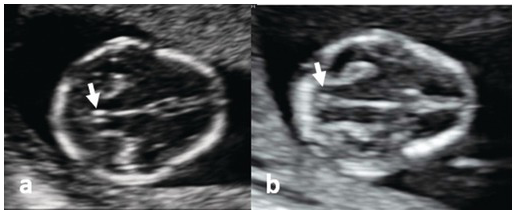
Fig. 1.15 (a) Transverse section of a normal fetal head, showing the thalami, cerebral peduncles and posterior fossa. The aqueduct is seen as a small slit (arrow) at a distance from the occiput. (b) Transverse section of the head in a fetus with spina bifida. The aqueduct is very close to the occiput (arrow) and is referred to as the ‘crash sign’.
Investigations
Offer invasive testing
Counselling
Absent IT as a sign of spina bifida or other pathology has a relatively high false-positive rate; therefore, a normal follow-up scan should be sufficiently reassuring
Intracranial Translucency Is Enlarged
Definition and Characteristics
99th centile for IT ranges from 3.0 mm (CRL 45–54 mm) to 3.4 mm (CRL 75–84 mm)
Ultrasound Assessment
Look for absent dividing septum between the IT (future fourth ventricle) and the cisterna magna
There is a high chance of posterior fossa abnormalities (Dandy–Walker malformation, inferior vermian hypoplasia/Blake’s pouch cyst) (Fig. 1.16)
Arachnoid cysts may look similar
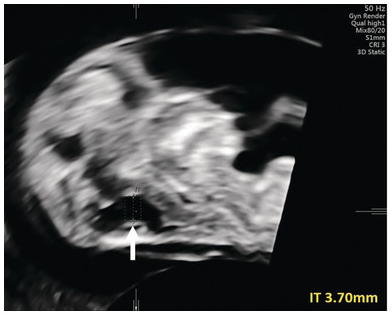
Fig. 1.16 Increased IT (arrow). A targeted scan at 16 weeks revealed a ‘classic’ Dandy–Walker malformation.
Investigations
Offer invasive testing for aneuploidies and genetic syndromes (e.g. Walker–Warburg syndrome)
Nasal Bone Is ‘Absent’
Definition and Characteristics
Ultrasound Assessment
Relies on comparison to overlying skin (Fig. 1.17)
‘Absent’ (unossified) nasal bone is less echogenic than skin and therefore only two dots are seen (Fig. 1.18)
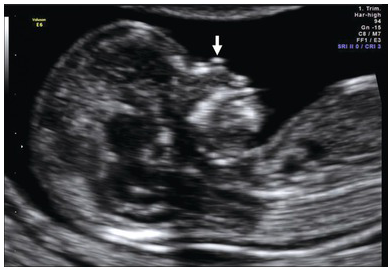
Fig. 1.17 Mid-sagittal view of the face showing an ossified nasal bone. Three ‘dots’ are visible – the brightest one is the nasal bone. Above the nasal bone is the skin; together they resemble = (equal sign). The third dot lying anteriorly is the tip of the nose (arrow). Note that the ultrasound beam is perpendicular to the nasal bone.
Investigations
If absent nasal bone has not been already included in the first trimester screening result, NIPT testing or karyotyping should be offered
If absent nasal bone has been included in the first trimester screening and the result is low risk, the risk is not recalculated in the second trimester
Counselling
When nuchal translucency and NIPT or karyotype are normal, interpret as a normal variant
The couple can be informed that there is no risk of cosmetic problems after birth
Micrognathia Is Suspected
Definition and Characteristics
Ultrasound Assessment
Visualise the retronasal triangle and mandibular gap on coronal section (Figs. 1.19 and 1.20)

Fig. 1.19 Coronal view of the face with retronasal triangle and characteristic gap between mandibular bones (arrow). This mandibular gap is a normal finding. In cases of micrognathia the chin is still visible but this mandibular gap can be absent.
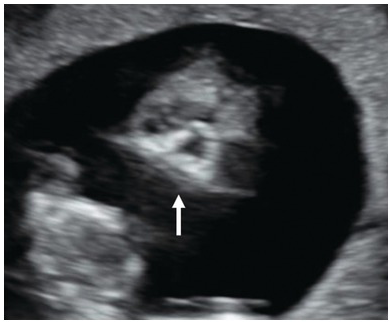
Fig. 1.20 In agnathia, no mandibular echogenicity is seen (arrow).
Investigations
Offer CVS when the retronasal triangle looks abnormal or mandibular gap is absent
Counselling
As false positives are relatively common, detailed ultrasound assessment should be repeated around 16–18 weeks
Cleft Lip and Palate Are Suspected
Definition and Characteristics
Cleft lip and palate are relatively common anomalies that are rarely suspected or detected during the first trimester scan
The incidence of cleft lip and palate is around 1 in 1,250, although it is more common in some countries (e.g. 1 in 500 in Japan)
Ultrasound Assessment
A cleft lip is recognised by lack of continuity of the upper lip in a coronal view (Fig. 1.21)
Maxillary gap in the mid‐sagittal view of the fetal face suggests that significant cleft palate is also present (Fig. 1.22)
Assessment of the retronasal triangle may reveal its abnormal shape or defects in the base of the triangle when cleft palate is present (Fig. 1.23)
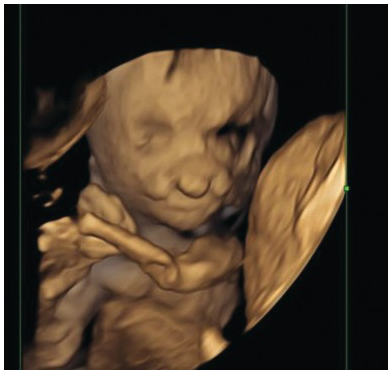
Fig. 1.21 3D picture of the face showing bilateral cleft lip.
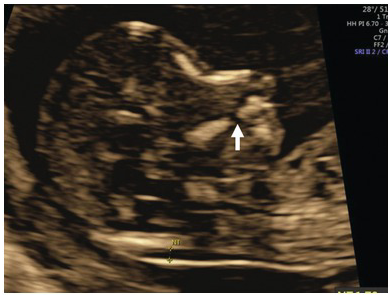
Fig. 1.22 Mid-sagittal view of the face showing a ‘gap’ in the maxilla which is indicative of cleft palate (arrow).
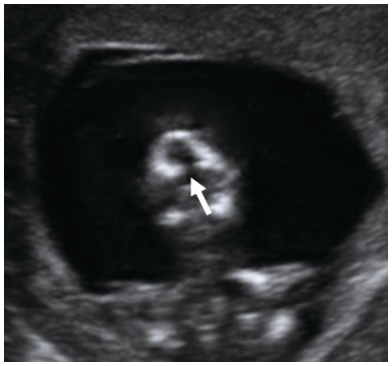
Fig. 1.23 Coronal view of the face of a fetus with cleft palate showing the retronasal triangle. The arrow points to the defect in the base of the triangle.
Investigations
Offer CVS when facial clefts are detected
Large Nuchal Translucency
Definition and Characteristics
Nuchal translucency (NT) is the subcutaneous accumulation of fluid behind the fetal neck (Fig. 1.24)
Typically identified during aneuploidy screening between 11+0 and 13+6 weeks of gestation (CRL 45–84 mm), it may also be identified before 11 weeks during pre-NIPT scans
Increased NT before 11 weeks is usually defined as >2.2 mm, corresponding to the 95th centile at 10 weeks
Raised NT before 11 weeks is best described as nuchal oedema to avoid confusion with ‘classic’ NT seen in a fetus with CRL > 45 mm after 11 weeks
NT ≥ 3.5 mm between 11+0 and 13+6 weeks, corresponding to the 99th centile, is associated with chromosomal abnormalities, genetic syndromes and a wide range of structural defects commonly involving cardiac, skeletal and lymphatic systems
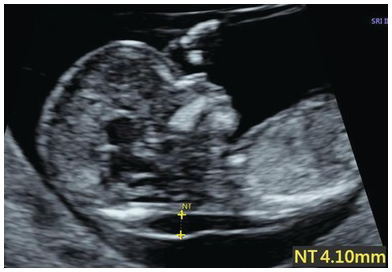
Fig. 1.24 Increased NT, no septa.
Ultrasound Assessment
Identify correct sagittal plane and position callipers correctly
Use colour Doppler to exclude nuchal cord (Fig. 1.25)
If views are suboptimal, transvaginal scan is recommended to exclude the presence of generalised subcutaneous oedema, mild ascites and pleural/pericardial effusions
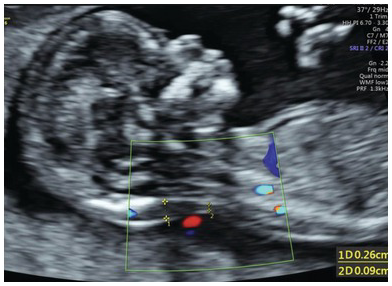
Fig. 1.25 NT with nuchal cord. Accurate measurement of the NT is compromised by the presence of nuchal cord. The measurements should be taken above and below the nuchal cord and the average used for risk calculation.
Investigations
Offer chromosomal microarray analysis
Large NT may also be an indication for exome sequencing – various cut-offs in terms of NT size have been proposed depending on local circumstances and availability of clinical geneticists to interpret variants of unknown significance
Counselling
Normal NIPT test may provide false reassurance by missing atypical aneuploidies
Structural abnormalities including hydrops may become apparent later in pregnancy (e.g. Noonan syndrome)
If karyotype and 20 weeks scan are normal, long-term outcome is comparable to the general population
Offer fetal echocardiography in the second trimester and a third trimester follow-up scan
Nuchal oedema seen before 11 weeks will resolve in ~80% of cases when reviewed again between 11 and 13+6 weeks, but the risk of an adverse outcome (chromosomal abnormality, major structural defect or miscarriage) remains relatively high (~10%)
Jugular Lymphatic Sacs
Definition and Characteristics
Jugular lymphatic sacs (JLS) are accumulations of lymphatic fluid in the anterolateral region of the fetal neck
Typically identified during anatomical survey of the neck in transverse section
Ultrasound Assessment
Use both sagittal and transverse plane and colour Doppler to distinguish from increased NT, septated cystic hygroma or nuchal cord
JLS will appear as spheroid echolucent ‘cysts’ in the anterolateral part of the neck (Fig. 1.26)
The sacs do not cross the midline posteriorly
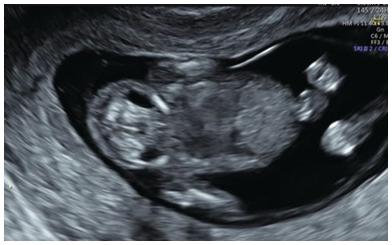
Fig. 1.26 Jugular lymphatic sacs are seen as small bilateral cystic areas on the sides of the neck.
Investigations
Offer invasive testing
Counselling
If microarray and NT are normal, it is likely to be a normal variant
If NT is increased but microarray analysis is normal, consider further testing for RASopathies (Noonan syndrome panel)
Cystic Hygroma
Definition and Characteristics
Cystic hygroma have cystic areas by the side of the neck, while increased NT is strictly confined to the back of the neck (Figs. 1.27 and 1.28)
The distinction between cystic hygroma and large NT is subjective – internal echoic structures are invariably present in both. Many experts will not attempt to make this distinction in the first trimester
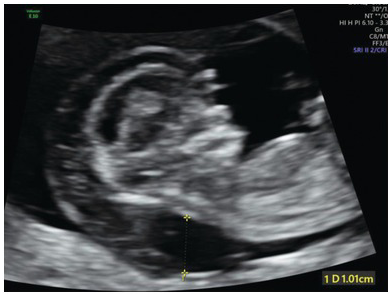
Fig. 1.27 Mid-sagittal view of the head and chest showing a large cystic hygroma. Generalised oedema is also present.
Ultrasound Assessment
Septation should be visible in the transverse plane
Fetal echocardiography should be performed as early as possible
Investigations
Offer microarray testing and, if available, testing for RASopathies (Noonan syndrome)
Counselling
Turner syndrome is the most common, but trisomies 21 and 18 and atypical chromosomal abnormalities are also represented
Turner syndrome with large hygroma and generalised oedema has very low survival rate (<5%)
Repeat fetal echocardiography in the second trimester and offer growth and wellbeing scans in the third trimester
~15–20% will have a good outcome with normal karyotype and normal paediatric follow-up
Heart
Anatomy Assessment
Ultrasound Assessment
Normal heart position with apex pointing to the left side of the chest (levocardia) (Fig. 1.29)
Normal four-chamber view (Fig. 1.30)
Normal three-vessel view/three vessels and trachea (3VV/3VT) view (Fig. 1.31)
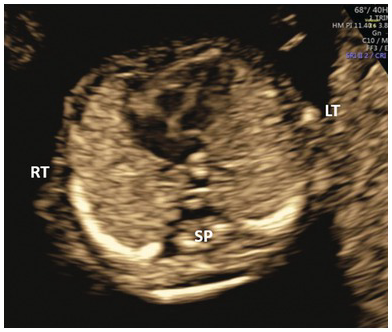
Fig. 1.29 Normal four-chamber view with the apex pointing to the left.
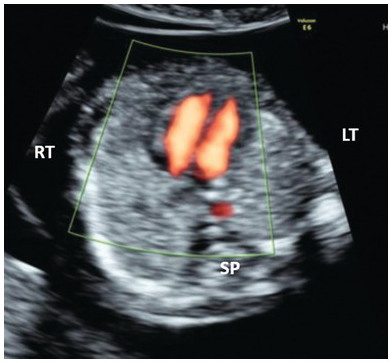
Fig. 1.30 Colour Doppler of the normal four-chamber view with blood flow reaching the apex of both ventricles.
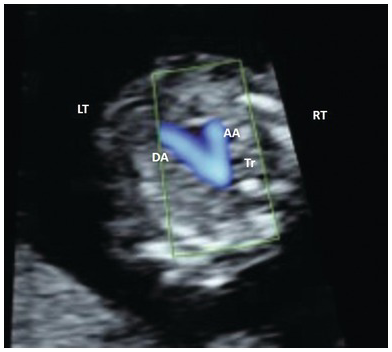
Fig. 1.31 Colour Doppler of the arches. Note that blood flow is towards the spine with both arches pointing to the left of the spine. AA, aortic arch; DA, ductal arch; Tr, trachea.
Significant Heart Abnormality Should Be Suspected If
Cardiac axis is abnormal
NT is enlarged
Tricuspid regurgitation is present (Figs. 1.32 and 1.33)
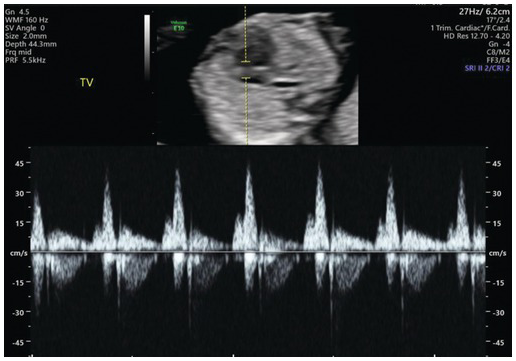
Fig. 1.32 Pulse wave Doppler showing normal flow across the tricuspid valve. Note that the sample volume is 3 mm and is placed across the whole tricuspid valve in an apical four-chamber view.
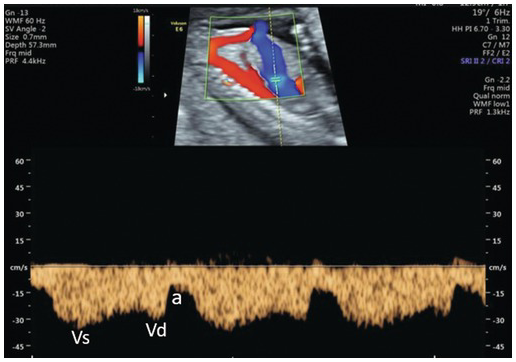
Fig. 1.34 Normal ductus venosus Doppler in the first trimester. The angle of the Doppler should be <20° in an adequately zoomed image with a sample volume of 0.5 mm placed at the colour aliasing point. In the typical ‘M’ waveform pattern the ‘a’ wave shows positive flow, which is normal. a, atrial systole; Vd, ventricular diastole; Vs, ventricular systole.
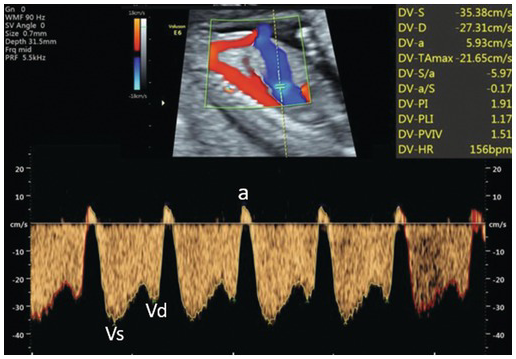
Fig. 1.35 Abnormal ductus venosus flow with reversal of the ‘a’ wave. a, atrial systole; Vd, ventricular diastole; Vs, ventricular systole.
Investigations
If significant cardiac anomaly is suspected, CVS for chromosomal microarray testing should be offered
Counselling
In most cases with normal microarray, the definitive diagnosis is best delayed until the second trimester
Abnormal Four-Chamber View
Univentricular heart; hypoplastic left heart syndrome (HLHS), tricuspid atresia, mitral atresia, severe coarctation Large atrioventricular septal defect Small left ventricle with unequal ventricular filling on colour Doppler (Figs. 1.36 and 1.37). Reversal of flow may be seen in the aortic arch (Fig. 1.38) Hypoplastic, hypokinetic right ventricle Hypoplastic right ventricle, large left ventricleUltrasound presentation Differential diagnosis Single ventricle Ventricular disproportion HLHS (Fig. 1.36), coarctation Pulmonary atresia Tricuspid atresia with ventricular septal defect Single channel of blood entering both ventricles Atrioventricular septal defect (Figs. 1.39 and 1.40)
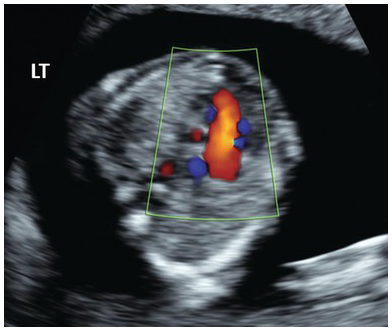
Fig. 1.37 Colour Doppler in HLHS shows single inflow filling on the right side and no filling on the left side.
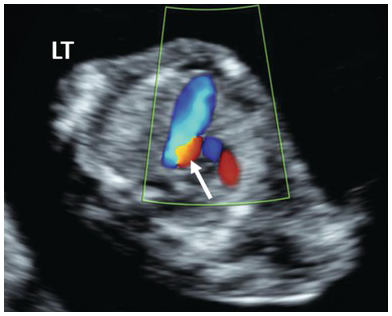
Fig. 1.38 Colour Doppler of the 3VT view in HLHS shows large pulmonary artery and reversal of flow in the aortic arch, shown in red (arrow).
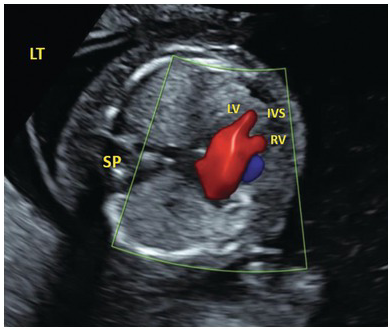
Fig. 1.40 Colour doppler in AVSD showing common atrioventricular flow. IVS, intraventricular septum; LV, left ventricle; RV, right ventricle; SP, spine.
Abnormal Three-Vessel View
Table 1.2 Abnormal three-vessel view
Large pulmonary artery, small aortic arch with reversed colour flow Hypoplastic left heart syndrome Small aortic arch with antegrade flow Coarctation Reverse flow in pulmonary artery and ductus venosus Pulmonary atresia with intact septum Small pulmonary artery with antegrade flow Tricuspid atresia with ventricular septal defect Single great vessel Transposition of great arteries (Figs. 1.41–1.44)
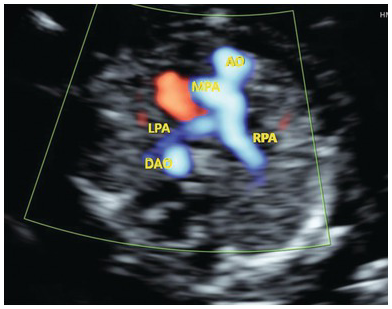
Fig. 1.42 Three-vessel view in TGA shows a cross section of the aorta anteriorly and the branching pulmonary artery posteriorly. In a normal fetus the anterior branching vessel is the pulmonary artery. AO, aorta; DAO, descending aorta; LPA, left pulmonary artery; MPA, main pulmonary artery; RPA, right pulmonary artery.
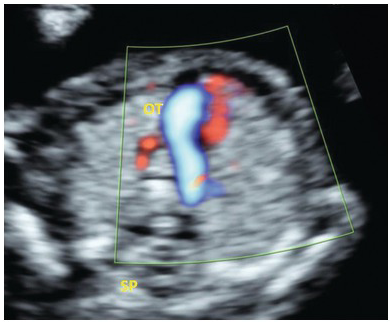
Fig. 1.43 Three-vessel trachea view in TGA shows a single outflow tract. The aorta is posterior and behind the PA and cannot be not seen at this level. OT, outflow tract; SP, spine.
Aberrant Right Subclavian Artery (ARSA)
Definition and Characteristics
Arises most distally from the aortic arch, goes behind the oesophagus and trachea to the right upper arm (Fig. 1.45)
The most common abnormality of the aortic arch
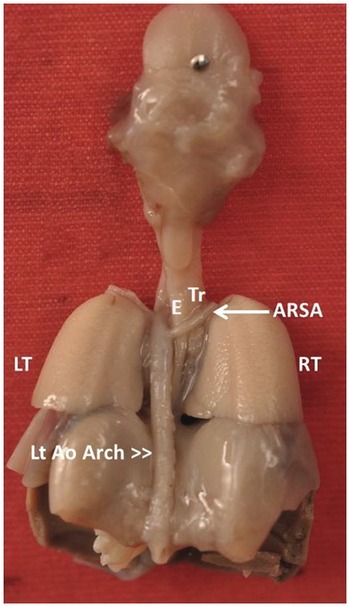
Fig. 1.45 Post mortem specimen viewed from the back showing the aberrant origin of the right subclavian artery (ARSA) from the aortic arch and traversing posterior to the oesophagus (E) and trachea (Tr).
Ultrasound Assessment
Measure NT
Examine fetal heart in standard planes
Exclude tricuspid regurgitation and abnormal ductus venosus
Colour Doppler PRF set to 0.9–1.8 KHz
Identify arches and aortic arch crossing trachea anterior and to the left of the spine (3VT view) (Fig. 1.46)
Move the probe cranially to identify clavicles
Normal subclavian artery is seen as a tortuous vessel going towards the clavicle and right arm
Aberrant right subclavian artery is seen coursing behind the trachea
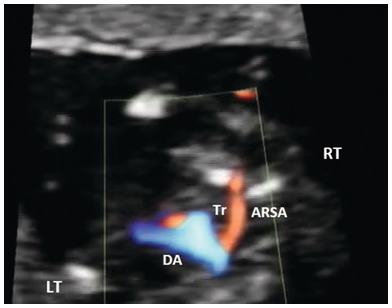
Fig. 1.46 Aberrant right subclavian artery (ARSA) seen in the 3VT view. DA, ductal arch; Tr, trachea.
Counselling
Isolated ARSA is not associated with an increased risk of aneuploidy
Fetal karyotyping including 22q11 deletion is advisable, if the background risk is higher or additional markers are present
Chest
Anatomy Assessment
Homogeneous lung echogenicity should be seen on both sides without pleural effusions or cystic or solid masses (Fig. 1.47)
Lungs are slightly more echogenic than cardiac muscles and fetal liver
Diaphragmatic continuity with normal position of stomach and liver (Fig. 1.48)
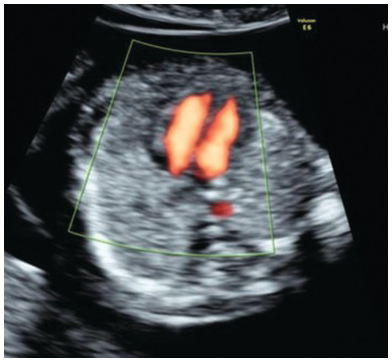
Fig. 1.47 Transverse section of the thorax at the four-chamber view with normal symmetrical lungs.
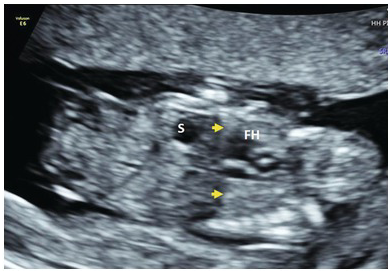
Fig. 1.48 Coronal view of the abdomen and thorax showing normal diaphragm (arrows). The stomach is seen below the diaphragm. FH, fetal heart; S, stomach.
Mediastinal Shift
Ultrasound Assessment
Mediastinal shift is diagnosed on a transverse four-chamber view plane by drawing an imaginary line connecting the spine and sternum, with the fetal heart lying on either side of the line
Congenital diaphragmatic hernia (CDH) should be suspected in the presence of mediastinal shift, abnormal cardiac axis or herniated stomach (Figs. 1.49 and 1.50)
Unilateral lung agenesis is also a (much rarer) possibility.
In the sagittal sections of the abdomen an abnormal course of the ductus venosus and ‘upturned’ course of the superior mesenteric artery are pointers point to CDH (Fig. 1.51)
In the coronal view the abdominal aorta is deviated
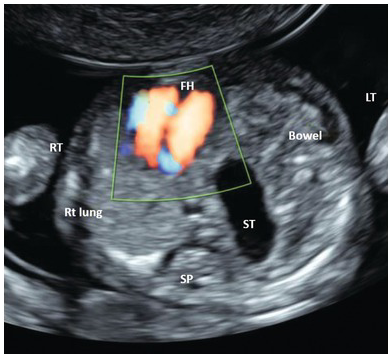
Fig. 1.49 Transverse section of the thorax showing left-sided diaphragmatic hernia. There is a mediastinal shift to the right. Stomach and small bowel are seen on the left side. FH, fetal heart; ST, stomach.
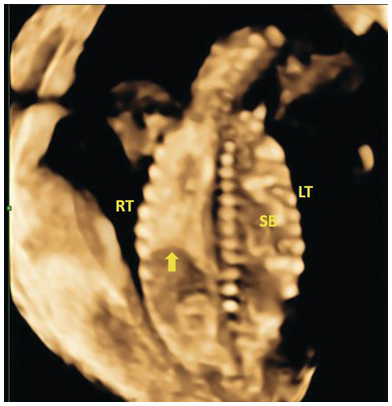
Fig. 1.50 Coronal 3D image of left diaphragmatic hernia with small bowel in the left hemithorax. The arrow points to the right hemidiaphragm. SB, small bowel.
Investigations
If CDH is suspected, offer invasive testing even if NT is normal; associated syndromes include trisomy 18, tetrasomy 12p (Pallister–Killian syndrome) and Cornelia de Lange syndrome
Counselling
Definitive diagnosis of intrathoracic pathology, including diaphragmatic hernia, is best delayed until the second trimester
Isolated unilateral lung agenesis may have a good outcome, but is more likely to be associated with congenital heart defects, including Scimitar syndrome (anomalous venous return from the right lung) with very high mortality rate
Pleural Effusion
Definitions and Characteristics
Ultrasound Assessment
Look for other evidence of hydrops (skin oedema, slight ascites)
Pericardial effusion can be mistakenly described as pleural effusion – the fluid in pericardial effusion surrounds the heart and is, therefore, seen on the medial aspect of the lung
Diagnosis should be confirmed on transvaginal scan
Unilateral effusions are very rarely seen in the first trimester
Investigations
Offer invasive testing (microarray)
Spine
Anatomy Assessment
Normal vertebral alignment in longitudinal, coronal and transverse views (Figs. 1.52–1.54)
Intact overlying skin
Particular effort should be made to confirm normal appearance of the spine when
– IT is absent
– biparietal diameter is <5th centile
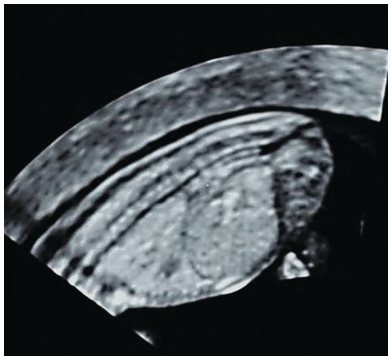
Fig. 1.52 Normal spine with intact skin posterior to the vertebrae from neck to sacrum in a mid-sagittal view. Note that vertebral bodies show ossification, but arches, which are still cartilaginous, are isoechoic or hypoechoic.
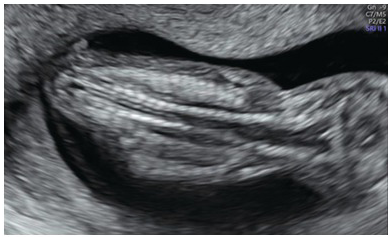
Fig. 1.53 Coronal view of the normal fetal spine at 13 weeks, showing normal cervical widening; the rest of the spine is parallel.
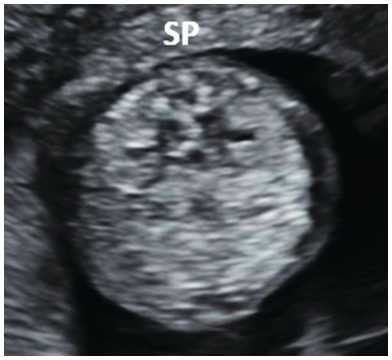
Fig. 1.54 Transverse view of the normal fetal lumbar spine of the same fetus (SP) showing the three ossification centres with intact overlying skin.
Meningomyelocele, Meningocele
Ultrasound Assessment
It is difficult to differentiate between meningocele and meningomyelocele as most first trimester defects are ‘flat’
Every effort should be made to determine whether the lesion is skin-covered (Fig. 1.55)
Investigations
Offer invasive testing if spinal abnormality is detected
Counselling
The outcome for open spina bifida is largely dependent on presence/absence of associated structural and chromosomal abnormalities, brain imaging later in pregnancy and the size and type of the lesion
In apparently isolated meningocele, it is best to delay the definitive diagnosis until second trimester
Even a large, thoracic skin-covered meningocele (limited dorsal myeloschisis) is associated with a very good long-term neurological outcome
Abdomen
Anatomy Assessment
A normal stomach filled with anechogenic gastric secretions is positioned in the left upper abdomen and is less echogenic than liver (on the right) (Fig. 1.56)
The empty fetal stomach has no clinical significance in the first trimester as most fetuses don’t swallow amniotic fluid before 14 weeks
The gall bladder is almost always seen by 14 weeks’ gestation, but non-visualisation in the first trimester has no clinical significance
With normal situs, the inferior vena cava is anterior and to the right of the descending aorta
Normal insertion of the umbilical cord should be documented after 12 weeks, together with the number of umbilical arteries
Signs of gastrointestinal obstruction have been reported in the first trimester (e.g. double bubble); however, definitive diagnosis should be delayed until the second trimester
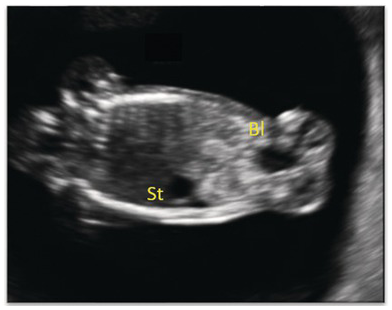
Fig. 1.56 Coronal view of fetal abdomen at 13 weeks, showing the stomach (St) and bladder (Bl).
Exomphalos
Definition and Characteristics
Exomphalos (outside the navel in Greek) and omphalocele (omphalos – umbilicus and cele – cavity) are synonyms
Prevalence of exomphalos is around 1 in 5,000 births
Small bowel herniation into a midline sac measuring <7 mm and seen before 13 weeks is likely to be a physiological midgut herniation
Ultrasound Assessment
Abdominal defect is membrane-covered (Figs. 1.57 and 1.58).
The umbilical cord arises from the dome of the sac
Large defects can include liver and stomach as well as bowel
Patent urachal cyst can be quite large and easily mistaken for an exomphalos
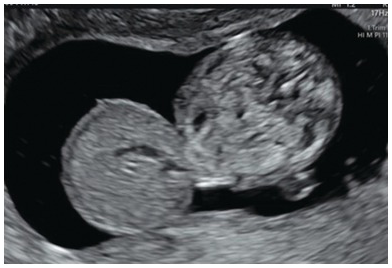
Fig. 1.57 Transverse view of the fetal abdomen showing a large anterior abdominal wall defect filled with liver. Note the covering membrane.
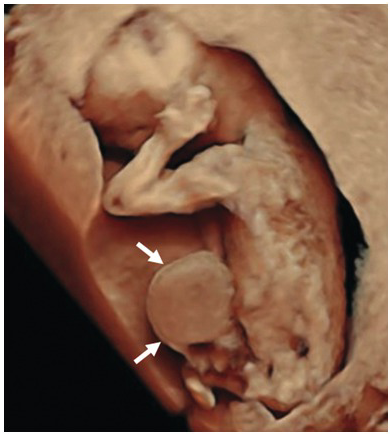
Fig. 1.58 3D image of the same fetus with exomphalos (arrows).
Investigation
Counselling
Small, isolated exomphalos with normal NT and normal karyotype will often resolve spontaneously in the late second trimester
If there is an isolated exomphalos with normal chromosomes, consider a possibility of Beckwith–Wiedemann syndrome; CVS material can be used for prenatal diagnosis by methylation analysis
Gastroschisis
Definition and Characteristics
A full-thickness paraumbilical abdominal wall defect to the right of the umbilicus
Prevalence of gastroschisis is rising, especially in young mothers where the prevalence has risen to 1 in 500
Ultrasound Assessment
The defect has no covering membrane (Fig. 1.59)
The defect is to the right of the normal cord insertion
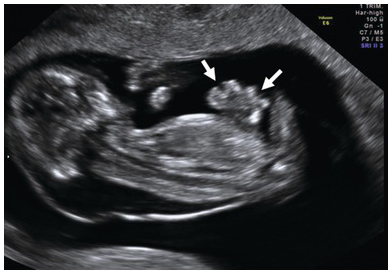
Fig. 1.59 Parasagittal longitudinal view of the fetus with an abdominal wall defect. Note the free-floating loops of bowel with no covering membrane (arrows).
Counselling
Apparently isolated gastroschisis is rarely associated with chromosomal and structural abnormalities and is, therefore, not an indication for invasive testing in the first trimester
Ectopia Cordis, Pentalogy of Cantrell or Body Stalk Anomaly?
Definitions and Characteristics
The original description by Cantrell includes five parts (pentalogy):
1. Deficiency of the anterior diaphragm
2. Midline suprapubic abdominal wall defect
3. Defect in the diaphragmatic pericardium
4. Cardiac abnormalities
5. Defect of the lower sternum
In ectopia cordis the heart is either partly or completely protruding through the sternal defect
Body stalk anomaly includes a large chest and abdominal wall defect with a very short or absent umbilical cord
Ultrasound Assessment
A combination of ectopia cordis with supraumbilical omphalocele points to the pentalogy of Cantrell – a complete Cantrell pentalogy has been rarely reported (Fig. 1.60)
In a body stalk anomaly, liver and bowel are often seen in the celomic cavity while an apparently intact amniotic sac contains the rest of the fetus. Umbilical cord is absent or very short with a baby in very close proximity to the placenta. Some degree of kyphoscoliosis is almost always present (Fig. 1.61)
Isolated ectopia cordis is very rare; therefore, the diagnosis should be confirmed in the second trimester
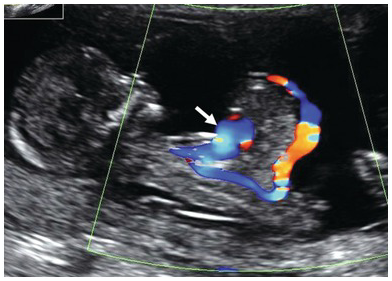
Fig. 1.60 Sagittal section of the fetus showing an abdominal wall defect with ectopia cordis (arrow).
Counselling
Body stalk anomaly is uniformly fatal, but fetal karyotype is usually normal
Pentalogy of Cantrell and ectopia cordis have a very high mortality even if chromosomes are normal
Genitourinary System
Anatomy Assessment
The fetal kidneys should be seen in a paraspinal location as bean-shaped, slightly echogenic structures with hypoechoic central renal pelvis
In the first trimester kidneys are better visualised in the coronal plane (Fig. 1.62)
Normal kidneys may appear hyperechoic
Colour doppler helps to identify renal arteries and the location of the kidneys (Fig. 1.63)
By 12 weeks’ gestation, the fetal bladder should be visible as a median hypoechoic round structure in the lower abdomen and measures <7 mm in longitudinal diameter
Colour Doppler can be used to confirm normal position of the bladder by identifying two umbilical arteries surrounding the bladder (Fig. 1.64)
Absence of bladder filling may be indicative of renal agenesis
Sex determination is 85% accurate and can be used in conjunction with cffDNA (cell-free fetal DNA) to ascertain the need for invasive prenatal diagnosis of X-linked conditions and management of congenital adrenal hyperplasia
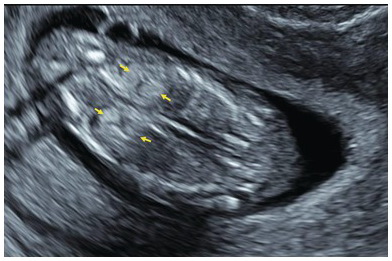
Fig. 1.62 Normal kidneys. Coronal view of the fetal abdomen showing normal kidneys, which are quite echogenic in the first trimester. Better visualisation can be achieved by increasing the dynamic range and by using ‘chroma’ colour.
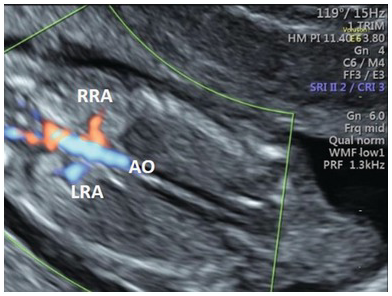
Fig. 1.63 Normal renal arteries. Coronal view of the fetal abdomen with colour Doppler showing both renal arteries. Ao, aorta; LRA, left renal artery; RRA, right renal artery.
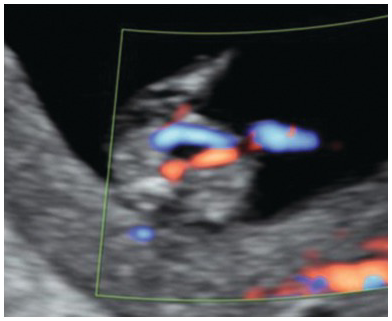
Fig. 1.64 Umbilical arteries. Colour Doppler of the lower abdomen showing a normal bladder surrounded by two umbilical arteries.
Bladder Is Large (Megacystis)
Definition and Characteristics
Usually defined as ≥7 mm in a longitudinal bladder diameter (LBD) (Fig. 1.65)
Prevalence in the first trimester is around 1 in 1,500–2,000
Around 40% of cases will be complex (chromosomal abnormalities, anorectal malformations or multiple anomalies)
40% will have a lower urinary tract obstruction (urethral atresia, stenosis or posterior urethral valve)
20% will resolve spontaneously
Ultrasound Assessment
Investigations
Offer invasive testing
Counselling
Megacystis when NT is >95th centile:
– high risk of complex pathology (chromosomal, anorectal or multiple abnormalities)
– isolated posterior urethral valve is very unlikely
Umbilical cord cyst is also present:
– High risk of a urethral atresia in both female and male fetuses
LBD < 12 mm with normal karyotype, normal NT and no cord cyst:
– spontaneous resolution is likely
– offer follow-up at 16, 20 and 28 weeks for reassurance
LBD > 12 mm, normal karyotype and NT, no cord cyst, male fetus:
– isolated posterior urethral valve is the most likely diagnosis
– Survival is around 50% and 25–30% of survivors will develop end-stage renal disease requiring dialysis and renal transplant by the age of 5
Skeletal System
Limb Abnormalities
Definition and Characteristics
Limb abnormalities detected in the first trimester can be isolated, but are more likely caused by chromosomal abnormality or a genetic syndrome (Table 1.3)
Terminal transverse defects are more common in the upper limbs; most of them are caused by a vascular injury or amniotic band sequence
Table 1.3 Limb abnormalities that can be detected in the first trimester
| Talipes equinovarous or clubfeet | In most cases the foot is twisted downward and inward |
| Clinodactyly | Abnormally bent finger due to abnormal bone development of the small bones of that finger |
| Clenched hand | Adducted thumb; second and fifth finger overlapping third and fourth |
| Polydactyly | Extra digits (Fig. 1.66) |
| Syndactyly | Two or more digits fused together |
| Ectrodactyly | Split hand or foot deformity with a deep central cleft (Fig. 1.67) |
| Phocomelia | Hands or feet attached close to the trunk |
| Sirenomelia | Fused lower limbs (Figs. 1.68 and 1.69) |
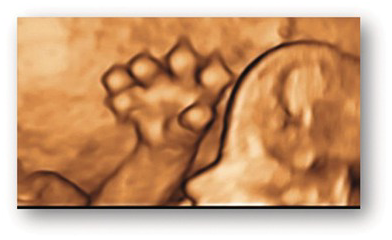
Fig. 1.66 Polydactyly. 3D image of a hand with post-axial polydactyly.
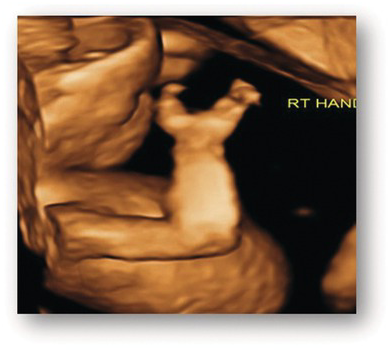
Fig. 1.67 Ectrodactyly. 3D image of a hand showing ectrodactyly (cleft hand).
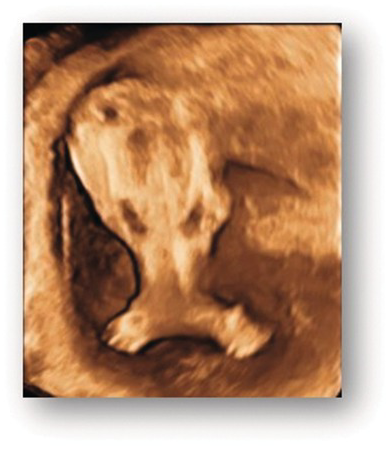
Fig. 1.68 3D image of fused lower limbs in sirenomelia (mermaid syndrome).
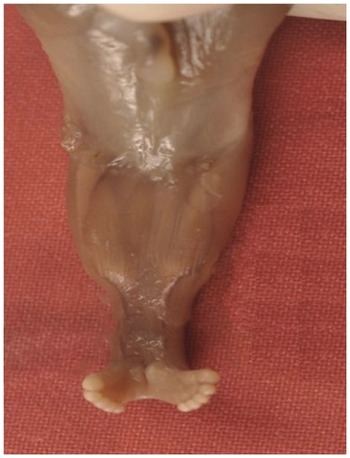
Fig. 1.69 Autopsy image of sirenomelia.
Ultrasound Assessment
Use transvaginal ultrasound to look for other abnormalities
Look for signs of amnion disruption
Investigation
Offer karyotyping/microarray
Counselling
The definitive diagnosis is best delayed until the second trimester
Short Femur
Definition and Characteristics
Femur length <5th centile
Ultrasound Assessment
A combination of short femur (<5th centile) and increased NT should prompt a detailed skeletal survey including transvaginal scan using the same principles as in the second trimester assessment (Table 1.4)
Table 1.4 Differential diagnosis when a short femur (<5th centile) is seen in combination with increased NT in the first trimester
| Additional phenotype | Differential diagnosis |
|---|---|
| Generalised oedema, hydrops | Achondrogenesis (Figs. 1.70–1.75), short rib polydactyly syndrome, Noonan syndrome |
| Poor skull ossification | Achondrogenesis, osteogenesis imperfecta, hypophosphatasia, boomerang dysplasia, Roberts syndrome |
| Small thorax | Achondrogenesis, Ellis-van Creveld syndrome, osteogenesis imperfecta, thantophoric dysplasia, campomelic dysplasia, hypophosphatasia, boomerang dysplasia, Jeune syndrome, short rib polydactyly syndrome |
| Polydactyly | Short rib polydactyly syndrome, Jeune syndrome, Ellis-van Creveld syndrome, acrocallosal syndrome |
| Oligodactyly | Roberts syndrome |
| Missing long bones | Boomerang dysplasia, Roberts syndrome, femoral facial syndrome |
| Hitchhiker’s thumb/toe | Atelosteogenesis, diastrophic dysplasia |
| Talipes | Campomelic dysplasia, hypophosphatasia, atelosteogenesis, diastrophic dysplasia, Roberts syndrome |
| Cardiac anomaly | Ellis-van Creveld syndrome, Meckel–Gruber syndrome, Noonan syndrome |
| Posterior fossa cyst | Ellis-van Creveld, Meckel–Gruber syndromes |
Fig. 1.71 2D image of the same fetus showing short upper limbs.
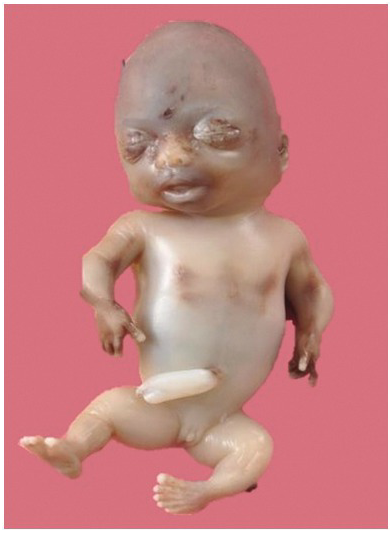
Fig. 1.72 Autopsy picture of the same fetus showing short limbs.
Fig. 1.73 Achondrogenesis at 13 weeks, showing significant oedema and poor mineralisation of the spine and skull.
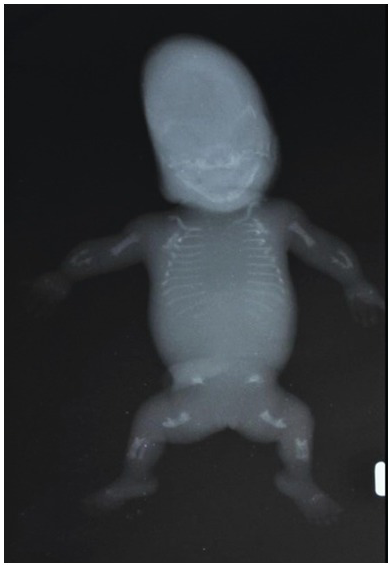
Fig. 1.74 X-ray of the same baby confirming poor ossification of the spine and skull.
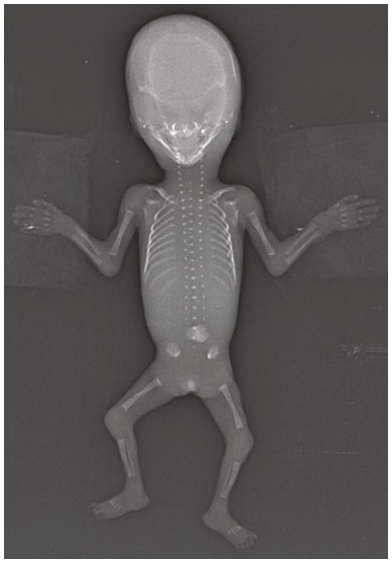
Fig. 1.75 Normal fetogram at 13 weeks. Note the distinct ossification of the spine and the normal long bones at this gestation.
Investigations
Short femur in combination with increased NT, or phenotype suggestive of skeletal dysplasia or genetic syndrome, is an indication for invasive testing
Counselling
If skeletal dysplasia is suspected, a definitive diagnosis based on the first trimester ultrasound appearances is best avoided and should be delayed until the second trimester
Increasingly, the definitive diagnosis can be made by targeted genetic testing (e.g. FGFR3 for suspected thanatophoric dysplasia)
Hydrops
Definition and Characteristics
Rather than using the umbrella term ‘hydrops’, it is more informative to be specific and describe a presenting phenotype more precisely; for example: pleural effusion, pericardial effusion, ascites, skin oedema, or a combination of these conditions (Fig. 1.76)
Virtually all cases presenting in the first trimester are non-immune; immune hydrops due to feto-maternal blood group incompatibility occurs after 15 weeks
Around 25% of cases presenting in the first trimester will have chromosomal abnormalities detected either by conventional karyotyping (20%) or microarray (additional 5%)
Exome sequencing can identify pathogenic genetic variants in up to 30% of otherwise unexplained cases
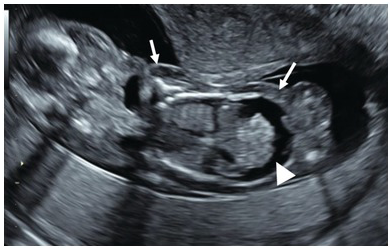
Fig. 1.76 Coronal section of fetus showing ascites (arrow head) and skin oedema (arrows).
Ultrasound Assessment
Detailed structural survey
Offer follow-up scan at 16 weeks to reassess anatomy with particular emphasis on fetal echocardiography and any changes in the amount of accumulated fluid
Investigations
Offer invasive testing, including exome sequencing if available
Maternal serology to exclude congenital viral infections (parvovirus B19, cytomegalovirus)
Counselling
Significant, progressive hydrops has poor prognosis; termination of pregnancy should be considered
Counselling for pathogenic genetic variants, if found, is complex and should involve clinical geneticists
Unexplained cases should be followed regularly during pregnancy, even when the 20-weeks scan looks entirely normal as reappearance in the third trimester is relatively common (e.g. undiagnosed Noonan syndrome)
In some cases of complete resolution in the second trimester, the definitive diagnosis has been established in early childhood (e.g. hereditary spherocytosis)
Placenta and Umbilical Cord
Anatomy Assessment
Single Umbilical Artery
Definition and Characteristics
Prevalence of single umbilical artery (SUA) is around 0.5%
Aplasia/atrophy has been suggested as the underlying cause
Laterality is of no clinical importance
Around 80% will be ‘isolated’
Ultrasound Assessment
Use a transverse plane at the level of the fetal bladder
Use colour Doppler mode to confirm the diagnosis (Fig. 1.77)
Use the transvaginal approach to assess anatomy
It is important to exclude renal agenesis
Measure NT
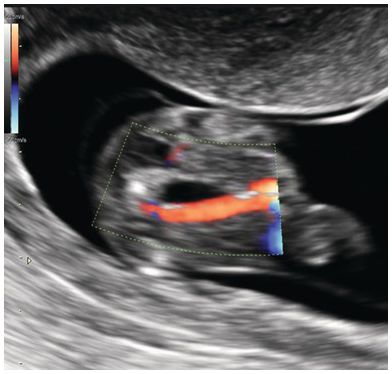
Fig. 1.77 Transverse section of fetal bladder. Colour Doppler shows an SUA.
Counselling
If SUA appears to be isolated and NT is normal, invasive testing is not indicated
Perinatal mortality is not increased
Detailed structural survey should be arranged in the second and third trimesters because of an increased risk of gastrointestinal atresias/stenosis and heart defects
If a third trimester scan (30–32 weeks) has confirmed that SUA is isolated, follow-up scans should be arranged to exclude fetal growth restriction
If there is no evidence of fetal growth restriction, early delivery is not indicated
Umbilical Cysts
Definition and Characteristics
Reported prevalence is higher (up to 3%) when transvaginal scans are used in the first trimester
Differentiation between true cysts and ‘pseudocysts’ with no epithelial lining (Wharton jelly cysts) is of little clinical value
Ultrasound Assessment
It is important not to describe the yolk sac (extra-amniotic cyst with echogenic borders) as an umbilical cyst
Urachal cyst should be suspected when there is a communication between bladder and extra-abdominal cystic structure surrounded by umbilical arteries (Fig. 1.78)
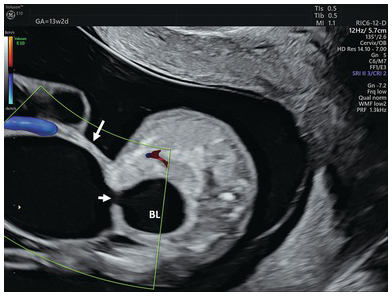
Fig. 1.78 Transverse section of the lower abdomen showing the bladder and a large urachal cyst in a fetus with trisomy 18. Note the communication (small arrow) between the bladder (BL) and a cyst (arrow).
Counselling
Isolated umbilical cord cysts seen in the first trimester have no clinical significance, regardless of their size or location (Fig. 1.79)
Even multiple umbilical cysts seen before 10 weeks are likely to disappear by 14 weeks
Detailed anatomy scan should be arranged around 16 weeks
In the absence of structural abnormalities, fetal karyotyping is not indicated
Extra-abdominal urachal cysts can be quite large and should not be mistaken for exomphalos or bladder exstrophy. If karyotype is normal, the prognosis after surgery is very good