⊙ Foundation of the Deep Carbon Observatory
Carbon is the fourth most abundant element in the universe. It is outweighed by hydrogen, responsible for nine-tenths of the mass of ordinary matter in the cosmos, and by helium. Hydrogen and helium are remnants of the Big Bang: they are products of the first three minutes of our fireworks universe. Oxygen, the third most abundant element, and carbon are ashes from the explosive finale of the evolution of stars.
In 2009, Robert (Bob) Hazen and his colleagues of the Carnegie Institution of Washington promoted the following connection between carbon in the universe and human existence on Earth:
Carbon plays an unparalleled role in human life. It is the element of life, providing the chemical backbone for all essential biomolecules. Carbon-based fuels supply most of society’s energy, while small carbon-containing molecules in the atmosphere play a major role in our variable and uncertain climate. Yet in spite of carbon’s importance scientists remain largely ignorant of the physical, chemical, and biological behavior of the carbon-bearing systems more than a few hundred meters beneath Earth’s surface.Reference Hazen, Hemley and Mangum1
Hazen et al. observed that we know neither how much deep carbon is stored in Earth’s interior as a whole nor how deep carbon migrates along the pathways between the reservoirs. Furthermore, our ignorance of the deep microbial system – that by some estimates rivals the total surface biomass – is profound. In short, our knowledge of deep carbon is seriously incomplete. To address this knowledge deficit, in 2009 the Carnegie Institution of Washington launched a decade-long program of research and discovery: the Deep Carbon Observatory (DCO). Its mission was to lay the groundwork for a new scientific discipline devoted to element number six – carbon – and its place in our lives and world. The emergence of this new collaboration in the geoscience community has changed how science is conducted across time zones, cultures and disciplines to bring global thinking to bear on the role and properties of carbon inside Earth.
In 2007, a chance encounter between Bob Hazen and Jesse Ausubel, a faculty member of Rockefeller University and a project officer at the Alfred P. Sloan Foundation, led to the concept of the DCO. Hazen, an accomplished writer of exhilarating science books at the cutting edge of research, was on a promotional tour. Hazen gave an after-dinner talk at the Century Association, a club for “congenial companions in a society of authors and artists” on Manhattan’s West 43rd Street. At this literary salon, Hazen spoke of his latest book, Genesis: The Scientific Quest for Life’s Origins, in which he suggested that geophysical reactions might have played a critical role in getting life started. Ausubel’s presence at this fundraising dinner was due to a last-minute cancellation by another participant. The presentation on the emergence of the first life on Earth made a deep impression on Ausubel. Hazen had developed a thinking style that envisaged life inevitably emerging as a consequence of chemistry, starting with water, organic molecules and a source of energy. His experiments in prebiotic chemistry showed the circumstances through which organic molecules could progress from structural simplicity to considerable complexity. This research was focused on how a prebiotic world rich in organic molecules could transition to the so-called RNA world of self-replicating genetic molecules. But above all, Hazen had emphasized the daunting gaps that existed in our knowledge of the origin of life and the special role of carbon. What Ausubel did next was to seek out Hazen’s book and read it.
Three months later, Ausubel contacted Hazen about the possibility of the Alfred P. Sloan Foundation supporting an integrated science approach to the pursuit of life’s origins. It would be a 10-year mission that drew on several branches of science – geology, biology, chemistry, physics and astronomy – in order to coordinate a multifaceted project to investigate early life on Earth and the role of the deep carbon cycle in its emergence. The first step was to convene an international workshop on the deep carbon cycle at the Carnegie Institution in May 2008. By this stage, Ausubel and Hazen were no longer focused solely on life’s origins: they felt that, in order to further human understanding of Earth and our place here, they needed to place the element carbon center stage. In his opening address at the three-day workshop, Hazen set out what he wanted the 110 participants to achieve:
A rare and important opportunity awaits us to define a new field. Collectively we need to assess what we don’t know. We will succeed in this endeavor if we accomplish three things, which I now charge all of you with. First, we have to look beyond our individual interdisciplinary expertise and see the subject in an integrated context: geology, chemistry, biology, physics – they are all going to play central roles. Second, we have to identify the key questions we want to have answered to understand the deep carbon cycle. That’s really what we’re here to do. And finally, we have to imagine what it’s going to take – what field observations, what key experiments, what new instruments, what theoretical advances are required to move this endeavor forward? I am tremendously excited to be here with you! I welcome you all! Let’s get started!
All three tasks contributed to the publication three years later of Carbon in Earth, a monumental book that is the benchmark for our present understanding of Earth’s carbon and a comprehensive review of what we already knew in 2009 and what we would have to learn in the DCO decade of discovery, 2009–2019.
This history of science book, From Crust to Core, complements Carbon in Earth by exploring four centuries of philosophical and scientific inquiry on the nature of Earth’s interior, its cycles and mechanisms and the particular roles of deep carbon. My aim has been to present a layered story of remarkably rich discovery. The narrative thread encounters about 150 pioneers of deep geoscience, several dozen research institutions and universities and more than 20 ships and research vessels. Many of these pioneers started their inquiries from points of view that at first sight might seem far away from the history of deep carbon science. On the other hand, such personal journeys of discovery are central to the philosophy of science, showing how science is actually done and the importance of asking the right questions and of understanding what the data are actually telling us.
⊙ Spheres Below and Heavens Above
Before commencing the historical narrative about the scientific discovery of Earth’s deep interior, I shall introduce the architecture of Earth from crust to core as we understand it today. Readers already familiar with the concepts may wish to skip this section. The first point to make is that everything that is deeper than about 10 kilometres is inaccessible to direct view. Furthermore, the temperature rises surprising quickly. In the world’s deepest gold mine, TauTona in South Africa, the temperature of the rock face is 60°C. At the bottom of the deepest borehole, 12 kilometres down, on the Kola Peninsula in Finland, the temperature reached 180°C, and which point further drilling became impossible.
Earth’s interior is divided into layers with different chemical compositions and mechanical properties. To picture the internal structure of Earth, we can begin with simple models. It can be likened to a stone fruit such as an avocado: both have a solid core surrounded by a thick mantle, with a crinkly surface skin or crust. For geophysical purposes, however, the core–mantle–crust model is too crude. To improve on that, we must think of Earth as being made up of a number of layers, like an onion: we can peel them off one by one, starting with the crust, below which there are two layers for the upper mantle and the lower mantle, separated by the transition zone between depths of 410 and 660 kilometres. The transition zones (or boundaries) are where phase changes occur in minerals as we proceed to greater pressures and higher temperatures. The boundary between the lower mantle and the outer core lies at a depth of 2900 kilometres. Below this is a liquid outer core, with a thickness of about 2300 kilometres, composed mainly of iron and nickel. The outer core is the seat of Earth’s magnetic field, which is generated through a self-induced dynamo process. The transition to a solid inner core is located 5100 kilometres below the surface. We’re going to examine these layers by working down from crust to core and then upwards from the surface to space.
Figure 1.1 shows the major divisions used in geophysics. The surface rocks are part of the crustal layer, which is rich in silica (silicon dioxide, SiO2). Its average thickness is about 38 kilometres beneath the continents and around 8 kilometres beneath the oceans. The five commonest elements in Earth’s crust are oxygen (47 percent), silicon (27 percent), aluminum (8.1 percent), iron (6.3 percent) and calcium (5 percent). Carbon (0.18 percent) is ranked tenth by its natural abundance in the crust. The thinner oceanic crust and the thicker continental crust are formed by entirely different processes and have different histories.
The crust and the uppermost mantle, considered as a mechanical entity, is known as the lithosphere (from the Greek lithos, meaning “rock”). It is the hard and rigid outer layer of Earth that has fractured into a dozen major plates (plus a handful of minor ones). Each tectonic plate is a layer of continental crust or oceanic crust supported by the viscous upper mantle. The oceanic lithosphere ranges in thickness from 50 to 150 kilometres, being at its thinnest at the mid-ocean ridges. The continental lithosphere is altogether a bulkier affair at a thickness of 40–280 kilometres or so, with a 30–50‑kilometre veneer of crust. The boundary between the crust and the mantle is referred to as the Moho, a convenient contraction derived from the name of the pioneering Croatian seismologist Andrija Mohorovičić (1857–1936). In 1909, he first noticed a discontinuity in the behavior of seismic waves crossing the Moho.
The 100–200‑kilometre-thick semi-fluid layer of hot plastic rock in the upper mantle is known as the asthenosphere (from the Greek asthenēs, meaning “weak”). Earth’s layers vary in thickness, mechanical strength and chemical composition. Actually, there are two different concepts of layering in the outer part of Earth: the crust and the mantle have different compositions (geochemistry), whereas the lithosphere and asthenosphere have different mechanical strengths (geophysics). Under the influence of long-term stress, the lithosphere exhibits rigidity, but deforms elastically and through brittle failure, whereas the asthenosphere deforms like a highly viscous fluid. The multiple layers of the mantle arise because as we go from crust to core we encounter ever-increasing temperatures and pressures. Minerals in the mantle adjust their atomic structures and chemical compositions in reaction to different temperature and pressure regimes. Such phase changes are detectable because they alter the velocities at which earthquake waves travel through the interior. A transition zone between 410 and 660 kilometres marks the boundary of the lower mantle and the upper mantle. Much of our knowledge of the mineralogy and composition of the mantle has also come from experiments with diamond anvil cells and from microscopic examination of inclusions in diamonds.
To complete this introductory survey of our dynamic planet, we need to rise above the interior and consider four interconnected spheres: lithosphere, hydrosphere, biosphere and atmosphere. Together these make up the complete system in which life on Earth exists. The system is a physical and biological domain that is the subject of many great debates on environmental issues, climate change and the origin and evolution of life. The hydrosphere encompasses the water on, under or above the surface. So, it includes the water in the oceans and seas, the liquid and frozen groundwater, the water locked in glaciers, icebergs and ice caps and the moisture in the atmosphere. Three-quarters of Earth’s surface is covered by oceanic saltwater – freshwater accounts for only 2.5 percent of Earth’s surface, and just a tenth of that is readily available from lakes, reservoirs and rivers. The hydrosphere is an intricate closed system in which water and other volatiles are continuously driven around in a cycle powered by solar energy and Earth’s gravity. This cycle moves water between the biosphere, atmosphere and lithosphere.
The atmosphere is the gaseous layer – commonly known as air – that surrounds Earth and is retained by gravity. By volume, dry air is 78 percent nitrogen, 21 percent oxygen, almost 1 percent argon and 0.04 percent carbon dioxide. Atmospheric scientists distinguish several layers in the atmosphere according to temperature and composition, as illustrated in Figure 1.2. The origin and evolution of the atmosphere is intimately connected to the interior dynamics of our planet. With the exception of the abundant oxygen released by photosynthesis, the atmospheric gases came from Earth’s interior and were released through volcanic eruptions. Carbon dioxide is abundant in volcanic gases, which raises the question: Which emits more carbon dioxide – Earth’s volcanoes or human activities? Terry Gerlach, a retired expert on volcanic emissions and formerly of the United States Geological Survey, estimates that leakage from volcanoes currently amounts to 0.15–0.26 billion tonnes of carbon dioxide a year, whereas the anthropogenic contribution is more than 100 times greater than that.Reference Gerlach2
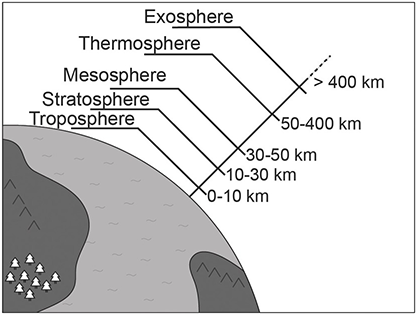
Figure 1.2 The principal divisions of Earth’s atmosphere.
Just one more sphere remains to complete the series: the magnetosphere, a magnetic envelope surrounding Earth. The atmosphere and magnetic field protect us from most radiation hazards. These hazards include high-energy particles spawned by violent astrophysical events throughout our galaxy, as well as ejections of mass from the solar corona and the particles in the solar wind that breeze past at velocities of 400 kilometres per second. The geomagnetic field exists thanks to a dynamo mechanism: heat flow from Earth’s inner core drives turbulent convection of fluid iron in the outer core, creating electric currents that produce a magnetic field. The mechanism is self-sustaining so long as there is sufficient energy to maintain convection.
⊙ Looking Down on Earth
To set the scene for this deep history, I shall begin in the period following World War II, when many physical scientists and engineers, exhausted by military research, returned to their laboratories and universities. What follows is one of several backstories leading up to the dramatic discovery of a dynamic Earth in the mid-1960s. In terms of the philosophy of science, I am including here a brief summary of the coincidental discovery of the fossil microwave radiation from the Big Bang event 13.7 billion years ago. During a few months in 1965, planetary and space science underwent enormous changes of human perspective – paradigm shifts, if you will. Twin revolutions of thinking upended our knowledge of the deep history of the universe and Earth and introduced a holistic systems approach to understanding how life, Earth and its environment, the universe and the natural world are interdependent and interlocked across multiple timescales.
In 1948, Fred Hoyle (1915–2001), an independently minded British astronomer and cosmologist, made this startling prediction in a radio broadcast:
Once a photograph of the Earth, taken from outside, is available … a new idea as powerful as any in history will be let loose.Reference Hoyle3
He later looked back with great pleasure on his forecast. On January 6, 1970, in his banquet speech to the First Lunar Science Conference in Houston, Texas, he proclaimed:
Well, we now have such a photograph. I’ve been wondering how this old prediction stands up. Has any new idea in fact been let loose? It certainly has … everybody has become seriously concerned to protect the natural environment. Something unique has happened to create an awareness of our planet as a unique and precious place.Reference Hoyle4
The first such photograph was a fuzzy 1966 monochrome image taken by the spacecraft Lunar Orbiter 1. The environmental game changer came two years later with the December 1968 Earthrise photograph taken during the Apollo 8 mission: Figure 1.3 shows our entire world as a small, blue, finite globe, with our nearest celestial neighbor, the Moon, a desolate presence in the foreground. Overnight the popular consciousness of people worldwide gasped at the fragility of our existence in the immensity of the cosmos. The perception of our isolation on a small world in orbit around an ordinary star profoundly altered human perceptions of our place in the universe. Humanity grasped the opportunity to find out how our planet functions as a whole system. Environmental movements compelled humans as a species to understand how we have reshaped the world. A new interdisciplinary field – Earth system science – flourished, nurtured by populist support. The 1970s saw the emergence of a new political ideology in many countries. As such, the nature photographer Galen Rowell promoted the image as “the most influential environmental photograph ever taken.” From 1990, Carl Sagan (1934–1996) famously championed the environmental context of extraterrestrial photographs of terra firma with Pale Blue Dot, a photograph of Earth taken by Voyager 1 from a distance of about 6 billion kilometres. So, when we first looked down on Earth from space, Hoyle's powerful new ideas were unleashed in the half-century from the mid-1960s to the present day.
Historically, 1963–1968 was a tumultuous period of revolutionary discovery in the Earth and space sciences. In 1963, Frederick Vine, a doctoral student of geophysics at Cambridge, together with his supervisor Drummond Matthews, put forward the hypothesis that sea-floor spreading at mid-ocean ridges could explain magnetic anomalies in the rocks of the ocean floor. The magnetism of the rocks had recorded the story of continents drifting apart, propelled by convective thermal energy from the deep. This seemed like madness at the time. Today we have a good understanding of the evolution of Earth, commencing with its hot formation about 4.6 billion years ago through accretion in the solar nebula. In early 1965, the progress of cosmology soared dramatically following the discovery of the cosmic microwave background: this fossil radiation is very dilute thermal energy from the hot Big Bang during the explosive origin of our universe 13.7 billion years ago. Hoyle praised the remarkable photograph from Apollo 8 five years after the astronomers’ discovery of a vast and dynamic universe above our heads and the geophysicists' discovery of a dynamic inner world beneath our feet.
⊙ The Invention of Earth System Science
The properties of our expanding universe are now so well established that a concordance cosmology emerged at the beginning of the twenty-first century. We have discovered with impressive precision the basic parameters of our universe, such as its age, its expansion rate and the properties of its mass and energy. And we are aware of a great deal about its contents: galaxies and clusters of galaxies, dark matter and dark energy, fossil radiation and gravitational waves, supermassive black holes, stars and interstellar gas, planets and their moons. We now have insights into how the various cosmic components evolve through time. These developments in astronomy became significant in the Earth sciences because it became possible to describe the history of matter, from the origin of the universe to the formation of the solar system. This theme is explored in Chapter 2.
The 50 years from Einstein's general theory of relativity in 1915 to the detection of the cosmic microwave background witnessed phenomenal progress in the philosophy of science. Cosmologists forged ahead, advancing from dreamy speculations about our universe being static or dynamic and arriving with total certainty at the conclusion that it is expanding. Observational astronomers broke out of the narrow bandwidth of the visible spectrum, swinging open new windows to the radio and X-ray universe. In the second half of the twentieth century, astronomers self-organized into large collaborations and networks, supported by public funding of space probes to the planets and great observatories in Earth orbit, such as the Hubble Space Telescope. By the dawn of the new century, the publication of data from large surveys and from space telescopes of all kinds had become open access, freely available to everyone.
While the astronomers probed ever further into outer space, the Earth scientists dived into our inner world. In a paradigm shift that mirrored what had happened in cosmology, new concepts developed rapidly, starting with plate tectonics. Yes, continents are adrift, with Europe and America distancing themselves by about 3 centimetres a year and India steadily crashing into Eurasia. A global recycling and reassembly scheme is in place. Steady subduction of the sea floor occurs at the continental margins, and the eruption of lava at the mid-ocean ridges marks the parting of the ways. Geoscience experienced a marked change of emphasis: geologists laid aside their hammers and instead embarked on ocean-going research ships to crack open the secrets of the hoary deep. Seismology had its shake-up, too, when exploration geophysics morphed from searching for hydrocarbon deposits of commercial interest to unlocking the details of Earth’s deep internal structure and dynamics.
The minerals in rocks tell us stories about their history: how, when and why they formed and to what extent they have been transported and transformed over deep time. To study how rocks and minerals behave deep down, geoscientists are able to replicate the high pressures and temperatures of Earth's interior in their laboratories. Remarkably, this can be accomplished at the lab bench with a thumbscrew and two diamonds. Here's how: in a diamond anvil cell, two brilliant-cut diamonds with perfectly flat faces are jammed head to head with a minute sample of rock between them. Diamond is such a hard form of carbon that it can withstand pressures up to hundreds of thousands of times (or more) greater than the atmospheric pressure at the surface.
From 1968, mineralogy expanded rapidly by combining the diamond anvil cell with the microscope in order to use a wide range of imaging and spectroscopic techniques to probe how the physical and chemical properties of minerals can change during geological processes. The study of minerals is a fundamental aspect of geophysical inquiry. Minerals existed before there were any forms of life on Earth. Indeed, minerals played an important role in the origin and evolution of life, interacting with biological systems in ways that we've only recently started to recognize. In the dynamic interior of our planet, the behavior of minerals as they churn their way through high-pressure and high-temperature regimes is the key to understanding the physical conditions in the deep Earth and how they have changed over geological time.
⊙ Environmentalists Shake Up Humanity
When we first looked back at Earth from space, James Lovelock, a British life scientist, was working for NASA. He collaborated on planetary exploration research with colleagues at the Jet Propulsion Laboratory (JPL), Pasadena, California. This was an exciting time at JPL: the Viking missions to Mars were to land two spacecraft safely on the red planet. Lovelock's job was to develop sensitive instruments for the analysis of the atmosphere and surface of Mars. This task was motivated in part to determine whether Mars supported life. In 1965, however, Lovelock took a hard look at the Martian atmosphere with its overwhelming abundance of carbon dioxide. Could that atmosphere really support life? Probably not, he concluded, noting its sharp contrast to the chemically dynamic mixture of nitrogen, oxygen and carbon dioxide in Earth's biosphere that brimmed with life.
Through his research for NASA concerning life on Mars, Lovelock formulated an audacious hypothesis. After discussions with Carl Sagan, Lovelock announced that the composition of Earth's atmosphere is largely a consequence – a by-product, as it were – of life on Earth. This Gaia hypothesis (or theory, as it is now known) is named after the Greek Earth goddess. Its claim is that life and its non-living environment is a self-regulating system that ensures Earth's climate and the composition of its atmosphere are always suitable for life to continue. It's an example of what cosmologists would later refer to, by turns, as fine-tuning or the anthropic principle; that is to say, the world is as it is because we are here to observe it. Lovelock developed this worldview in the 1960s and 1970s through cooperation with the American evolutionary theorist Lynn Margulis (1938–2011). The Gaia theory is the first scientific statement of Earth as a symbiotic system that is more than the sum of its parts. Lovelock was familiar with systems theory and cybernetics. As a result, he had a high level of familiarity with positive and negative feedback. He felt that a system as complex as Earth must have a multitude of such loops. From this point, the identification of feedback loops in Earth's natural systems became a key driver of Earth system science.
The Gaia theory did not gain deep and wide acceptance in the scientific community, probably because associating it with Greek mythology and philosophy was a serious category error, but it did capture public interest. It boosted the environmental movement that really took off in the USA largely thanks to the advocacy of scientists such as marine biologist Rachel Carson (1907–1964). Her book Silent Spring (1962) dealt with environmental problems that she believed were caused by synthetic pesticides such as dichlorodiphenyltrichloroethane (DDT). The overriding theme of Silent Spring is the powerful – and often negative – impact humans have on the natural world. Hers was the first voice to spread concern that the human use of chemicals could interfere with the biosphere on a global scale.
Other areas of concern about pollution of the Earth system rapidly followed. Lovelock's laboratory research led to him becoming the first to detect the global build-up of chlorofluorocarbons (CFCs) in the stratosphere. CFCs are organic compounds containing carbon, chlorine and fluorine. They were widely used as refrigerants, aerosol propellants and solvents. In 1974, two atmospheric chemists, Mario Molina and Sherwood Rowland (1927–2012), found that CFCs were depleting the protective ozone layer in the stratosphere that absorbs most of the Sun's ultraviolet radiation: long-lived chlorine ions react with ozone to form long chains of molecules composed of chlorine and oxygen. They received the Nobel Prize in Chemistry in 1995 for this discovery. By 1987, the Montreal Protocol set strict limits for CFC emissions. Even so, it will take until 2075 for the polar ozone layer to recover completely. Our use before the 1980s of refrigerants based on carbon and halogens has changed the chemical make-up of the stratosphere to such an extent that full recovery of the Earth system will take nearly a century. In the 1980s, rising atmospheric pollution by another gas, carbon dioxide, also led to calls for global action to mitigate the great global warming crisis.
⊙ Global Warming and Deep Carbon
By the middle of the nineteenth century, a few scientists were aware that climate change must have occurred, simply to explain Ice Ages. The outstanding Irish physicist John Tyndall (1820–1893) was a keen alpinist: he was among the first to ascend the Weisshorn (4506 m) and was an early climber of the Matterhorn (4478 m). His familiarity with glaciers, on which he wrote a major monograph, convinced him that tens of thousands of years ago northern Europe must have been entirely buried under colossal amounts of ice, and therefore the climate must be subject to long-term change. He was aware that Joseph Fourier (1768–1830) in Paris had suggested in 1824 that Earth’s atmosphere kept the surface temperature warm by acting as a blanket blocking transfer of the Sun’s radiant heat to the vacuum of space.Reference Fourier5 As a keen experimentalist at London’s Royal Institution, Tyndall decided to find out if there were any gases in the atmosphere that could trap infrared radiation. And yes, there were three: he identified water vapor as the most important, but he also found that carbon dioxide and methane as trace gases are amazingly effective at altering the balance of heat radiation through the entire atmosphere.
Towards the end of the nineteenth century, Swedish physical chemist Svante Arrhenius (1859–1927) became fascinated by the riddle of the historical cause of Ice Ages, which had become a hotly contested topic at the Stockholm Physical Society. He was the first scientist to model the effect of atmospheric carbon dioxide on global warming. His model suggested that doubling the amount of carbon dioxide would raise the temperature in Europe by 5–6°C.Reference Arrhenius6 His colleague Arvid-Gustave Högbom (1857–1940), also at the University of Stockholm at the time, had a strong interest in the global carbon cycle. He estimated that the flux of carbon dioxide from the industrial burning of coal (a form of deep carbon) was comparable to that from natural geochemical sources such as volcanoes. In a popular book published in 1908, Arrhenius suggested that a doubling of carbon dioxide would take many centuries. In any case, the concept of warming was naturally attractive to Scandinavian scientists, and the German physicist Walther Nernst (1864–1941) even suggested setting fire to unused coal seams to boost the global temperature. Tyndall, Fourier, Arrhenius and Högbom all contributed to the study of the effect of carbon dioxide atmospheric thermodynamics as an important aspect of Earth system science. However, the realization that anthropogenic carbon dioxide emissions would have massive implications for the future habitability of the planet did not come in their lifetimes.
In 1958, Charles David Keeling (1928–2005), an early career scientist at the Scripps Institute of Oceanography in San Diego, invented a detector for sniffing the air and measuring the concentration of carbon dioxide. On November 22, 1958, he set in train the daily monitoring of carbon dioxide outgassing on the north flank of Mauna Loa, the largest volcano in the world, topping out at 3397 m and at a great distance from major sources of pollution. The Mauna Loa Observatory has continued hourly measurements of atmospheric quality ever since. Keeling’s initial results gave a concentration of 310 parts per million (ppm). When he died this number had risen to 380 ppm, and at the time of writing (2018) it has soared to 420 ppm. On April 25, 1969, Keeling took his findings of the rise in carbon dioxide to a symposium at the American Philosophical Society on the long-term medical implications of atmospheric pollution.Reference Keeling7 His paper was preceded by one on lead pollution and followed by one on air pollution and plant life. He was writing at a time of severe smog pollution in Los Angeles and San Diego, which had adversely affected him and his family. Here’s the final sentence from his paper:
If the human race survives into the twenty-first century with the vast population increase that now seems inevitable, the people living then … may face the threat of climate change brought about by an uncontrolled increase in atmospheric CO2 from fossil fuels.
Historically, that’s one of the first hard-hitting statements about the unexpected global consequences of the accelerated use of deep carbon as a source of energy for industrial and domestic purposes.
⊙ Jules Verne Imagines Earth’s Dynamic Interior
In concluding this chapter, I would like to comment on Jules Verne’s second epic adventure novel, the scientific romance Voyage au centre de la Terre, published by Pierre-Jules Hetzel in 1864. It is an impressive example of the use of science fiction to promote the latest discoveries and their possible applications. In Verne’s day, divergent theories about the nature of Earth had scholars disputing whether Earth’s interior was liquid or solid. Estimates of the age of Earth were subject to stupendous variations. Darwin’s theory of biological evolution over immense periods of time, together with the geological evidence for the development of Earth over countless millions of years, challenged the literal descriptions of creation in biblical texts. In the mid-nineteenth century, a curious public and enthusiasts became excited by public lectures and popular science expositions on Earth’s distant history. Dinosaur fossils began to be displayed in museums. Verne, and other writers, tapped into this interest in the past by crafting engaging narratives in which they shared controversial ideas about extinction, evolution and a world once dominated by reptiles.
Throughout Voyage au centre de la Terre, Verne’s familiarity with the geoscientists of his day and their theories shines through impressively, thanks to his scrupulous research. Verne’s inspiration came from Scottish geologist Charles Lyell (1797–1875), who had investigated volcanoes and earthquakes. Verne learned about volcanic phenomena from Charles Sainte-Claire Deville (1814–76), a geologist at the Collège de France, who had made a geological study of Tenerife (1848) and had witnessed eruptions of Stromboli, the setting for the conclusion of the novel. Verne cites Georges Cuvier (1769–1832) five times in connection with his research on fossils that established the extinction of species as a fact, as well as Cuvier’s opposition to evolution.








