Chronic obstructive pulmonary disease (COPD) remains a potent public health problem in the USA and globally( Reference Mannino and Buist 1 , Reference Lozano, Naghavi and Foreman 2 ). After decades of increased mortality from COPD in the USA, the rate of mortality has stabilised since 2000( Reference Ford, Croft and Mannino 3 ). Although the prevalence of smoking is the chief driver of the rate of mortality from COPD, identifying other factors that may influence mortality and are amenable to interventions in individuals with COPD is of substantial interest.
Studies, mostly cross-sectional ones, have related nutritional factors including the intake of fruits and vegetables, which are rich sources of antioxidants, to pulmonary function and COPD( Reference Strachan, Cox and Erzinclioglu 4 – Reference Hirayama, Lee and Binns 14 ). Dietary intakes of vitamin C( Reference Schwartz and Weiss 15 – Reference Chen, Tunstall-Pedoe and Bolton-Smith 19 ), vitamin E( Reference Dow, Tracey and Villar 20 , Reference Schunemann, McCann and Grant 21 ), β-carotene( Reference Grievink, Smit and Ocke 18 , Reference Chen, Tunstall-Pedoe and Bolton-Smith 19 , Reference Chuwers, Barnhart and Blanc 22 ) and carotenoids( Reference Schunemann, McCann and Grant 21 ) have been linked to lung function. Furthermore, circulating concentrations of antioxidants including carotenoids, vitamin C, vitamin E and vitamin A have been linked to pulmonary function in cross-sectional studies( Reference Van Antwerpen, Theron and Richards 23 – Reference Semba, Chang and Sun 31 ) and to change in pulmonary function in prospective studies( Reference Alfonso, Fritschi and de Klerk 32 – Reference Guenegou, Boczkowski and Aubier 34 ).
Assuming that adequate nutritional status may help to preserve lung function and that preserved lung function reduces mortality, adequate nutritional status might reduce mortality among people with impaired lung function. An ecological study has suggested that increased intakes of fruits and fish are inversely correlated with COPD mortality( Reference Tabak, Feskens and Heederik 35 ). Prospective studies have found that the intakes of fruits and vitamin E are inversely associated with mortality from COPD( Reference Walda, Tabak and Smit 36 ). Yet, little is known about the associations between antioxidant concentrations and mortality in adults with obstructive lung function. Oxidation is considered to be a key factor in the pathogenesis of COPD( Reference MacNee 37 – Reference Biswas, Hwang and Kirkham 39 ), but very little is known about the role of oxidation in and any possible beneficial effects of antioxidants on the prognosis of this disease. Therefore, the objective of the present study was to examine the associations between circulating concentrations of carotenoids (α-carotene, β-carotene, cryptoxanthin, lutein/zeaxanthin, and lycopene), vitamin C, vitamin E, and Se and all-cause mortality in a cohort of US adults with obstructive lung function.
Methods
The present study is based on data obtained from the Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality Study (baseline examination from 1988 to 1994; mortality follow-up through 2006)( 40 , 41 ). A complex sampling design (stratified multistage probability design) was used to select participants, who constituted a representative sample of the civilian non-institutionalised population in the USA. The participants were interviewed at their homes and extended an invitation to undergo an examination in the mobile examination centre, where they completed additional questionnaires, underwent a series of examinations, and provided blood and urine samples. The interview and examination response rates were 86 and 78 %, respectively. NHANES III received approval from the Institutional Review Board.
A probabilistic match of participants’ information with the National Death Index death certificate records was conducted to identify deceased participants. If a match was not made, the participant was assumed to be alive at the end of the follow-up period. International Classification of Diseases-10 codes I00–I99 and C00–C97 were used to define deaths from circulatory disease and cancer, respectively.
Adults were eligible for a pulmonary function test without post-bronchodilator testing. The procedures used to conduct spirometry have been detailed elsewhere( 42 ). The following equations were used to calculate predicted forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) in adults:
FEV1:
 $$\begin{eqnarray} White\ men:\ 0.5536 - 0.01303\times age - 0.000172\times age^{2} + 0.00014098\times height^{2},\quad African\ American\ men:\ 0.3411 - 0.02309\times age + 0.00013194\times height^{2},\quad Mexican\ American\ men:\ 0.6306 - 0.02928\times age + 0.00015104\times height^{2},\quad White\ women:\ 0.4333 - 0.00361\times age - 0.000194\times age^{2} + 0.00011496\times height^{2},\quad African\ American\ women:\ 0.3433 - 0.01283\times age - 0.000097\times age^{2} + 0.00010846\times height^{2},\quad Mexican\ American\ women:\ 0.4529 - 0.01178\times age - 0.000113\times age^{2} + 0.00012154\times height^{2}. \end{eqnarray}$$
$$\begin{eqnarray} White\ men:\ 0.5536 - 0.01303\times age - 0.000172\times age^{2} + 0.00014098\times height^{2},\quad African\ American\ men:\ 0.3411 - 0.02309\times age + 0.00013194\times height^{2},\quad Mexican\ American\ men:\ 0.6306 - 0.02928\times age + 0.00015104\times height^{2},\quad White\ women:\ 0.4333 - 0.00361\times age - 0.000194\times age^{2} + 0.00011496\times height^{2},\quad African\ American\ women:\ 0.3433 - 0.01283\times age - 0.000097\times age^{2} + 0.00010846\times height^{2},\quad Mexican\ American\ women:\ 0.4529 - 0.01178\times age - 0.000113\times age^{2} + 0.00012154\times height^{2}. \end{eqnarray}$$
FVC:
 $$\begin{eqnarray} White\ men:\ - 0.1933 + 0.00064\times age - 0.000269\times age^{2} + 0.00018642\times height^{2},\quad African\ American\ men:\ - 0.1517 - 0.01821\times age + 0.00016643\times height^{2},\quad Mexican\ American\ men:\ 0.2376 - 0.00891\times age - 0.000182\times age^{2} + 0.00017823\times height^{2},\quad White\ women:\ - 0.3560 + 0.01870\times age - 0.000382\times age^{2} + 0.00014815\times height^{2},\quad African\ American\ women:\ - 0.3039 + 0.00536\times age - 0.000265\times age^{2} + 0.00013606\times height^{2},\quad Mexican\ American\ women:\ 0.1210 + 0.00307\times age - 0.000237\times age^{2} + 0.00014246\times height^{2}. \end{eqnarray}$$
$$\begin{eqnarray} White\ men:\ - 0.1933 + 0.00064\times age - 0.000269\times age^{2} + 0.00018642\times height^{2},\quad African\ American\ men:\ - 0.1517 - 0.01821\times age + 0.00016643\times height^{2},\quad Mexican\ American\ men:\ 0.2376 - 0.00891\times age - 0.000182\times age^{2} + 0.00017823\times height^{2},\quad White\ women:\ - 0.3560 + 0.01870\times age - 0.000382\times age^{2} + 0.00014815\times height^{2},\quad African\ American\ women:\ - 0.3039 + 0.00536\times age - 0.000265\times age^{2} + 0.00013606\times height^{2},\quad Mexican\ American\ women:\ 0.1210 + 0.00307\times age - 0.000237\times age^{2} + 0.00014246\times height^{2}. \end{eqnarray}$$
Mild obstructive impairment was defined as a FEV1/FVC < 0·70 and a FEV1 ≥ 80 %, moderate obstructive impairment was defined as a FEV1/FVC < 0·70 and a FEV1 50 to < 80 % predicted, and severe obstructive impairment was defined as a FEV1/FVC < 0·70 and a FEV1 < 50 % predicted. Participants with mild, moderate or severe COPD were combined for analyses that examined associations between antioxidant concentrations and mortality risk.
The serum concentrations of five carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene), vitamin C and α-tocopherol were measured using an isocratic reversed-phase HPLC. The serum concentrations of Se were measured using graphite furnace atomic absorption spectroscopy.
The following covariates were included in the analyses: age; sex; self-reported race or ethnicity (white, African American and other); educational level ( < 12, 12, or >12 years); smoking status; alcohol consumption; leisure-time physical activity; use of vitamin or mineral supplements; systolic blood pressure; HDL-cholesterol; non-HDL-cholesterol; BMI; C-reactive protein; urinary albumin:creatinine ratio; health status; histories of myocardial infarction and stroke; diabetes. Sociodemographic and lifestyle variables were included in the analyses because these variables are generally strongly associated with mortality. Furthermore, these variables are also associated with antioxidant concentrations. Lipid concentrations were included in the analyses because many of the antioxidants are lipid soluble. C-reactive protein as a marker of inflammation was included in the analyses because sources of inflammation can reduce antioxidant concentrations. Systolic blood pressure, BMI, urinary albumin:creatinine ratio, health status, diabetes, and histories of myocardial infarction and stroke were included in the analyses because these risk factors and conditions predict mortality and are associated with antioxidant concentrations.
Participants who reported that they had smoked at least 100 cigarettes during their lifetime and that they still smoke were designated as current smokers. Participants who reported that they had smoked at least 100 cigarettes during their lifetime and that they no longer smoke were designated as former smokers. Participants reporting not having smoked at least 100 cigarettes were designated as never smokers. Using information from a FFQ, the frequency of alcohol consumption per month was estimated. Participants who engaged three or more times per week in an activity with a metabolic equivalent level ≥ 6 (for those aged ≥ 60 years) and with a metabolic equivalent ≥ 7 (for those aged < 60 years) were considered to be vigorously active, participants who engaged five or more times per week in activities of which no more than two could be considered vigorous were considered to be moderately active, participants who engaged in activities that were not vigorous or moderate were considered to be lightly active, and participants who engaged in no leisure-time physical activity were considered to sedentary. Use of any vitamins or minerals by the participants was determined from the responses provided to the question ‘Have you taken any vitamins or minerals in the past month?’
The second and third systolic blood pressure measurements were averaged. BMI (kg/m2) was calculated from the measured weight and height. The concentrations of HDL-cholesterol were measured on a Hitachi 704 analyser (Boehringer Mannheim Diagnostics) after precipitation of other lipoproteins with a heparin–manganese chloride mixture. The concentrations of non-HDL-cholesterol were calculated by subtracting the concentration of HDL-cholesterol from that of total cholesterol. The serum concentrations of C-reactive protein were measured using latex-enhanced nephelometry on the Behring Nephelometer Analyzer System (Behring Diagnostics, Inc.). The urinary concentrations of albumin were measured using a fluorescent immunoassay on a Sequoia-Turner fluorometer (Sequoia-Turner Corporation). The urinary concentrations of creatinine were measured from the rate of colour formation on a Beckman Synchron AS/ASTRA clinical analyser (Beckman Instruments, Inc.).
Participants with a history of myocardial infarction or stroke were identified from the responses provided to the questions ‘Has a doctor ever told you that you had a heart attack?’ and ‘Has a doctor ever told you that you had a stroke?’, respectively. Participants who provided a positive response to the question ‘Have you ever been told by a doctor that you have diabetes or sugar diabetes?’ or had glycated Hb concentration ≥ 6·5 % were considered to have diabetes.
Men and non-pregnant women aged 20–79 years who underwent a spirometric examination in the mobile examination centre and had reproducible FEV1 and FVC results were included in the analyses. Using the direct method, age adjustment was done to the projected year 2000 US population for adults aged 20–79 years. Least-squares adjusted mean concentrations of antioxidants were calculated. Proportional-hazards analysis was used to estimate hazard ratios for antioxidants and mortality. In these multivariable models, antioxidants were modelled as continuous variables and again as quintiles calculated from the distributions of antioxidants among participants with obstructive lung function. To better approximate a normal distribution of several antioxidants, the following transformations were used in regression analyses: log transformation for β-carotene, lutein/zeaxanthin, lycopene, total carotenoids, and vitamin E and square root transformation for vitamin C. SAS (SAS Institute Inc.) and SUDAAN (Research Triangle Institute) were used to conduct statistical analyses.
Results
Of the 15 033 men and non-pregnant women aged 20–79 years who visited the mobile examination centre, 14 082 had reproducible FEV1 and FVC results. After exclusion of participants with an unreliable examination result, 13 134 remained. Of these participants, 1746 had obstructive lung function and 12 168–12 516 participants had a measurement of the antioxidants. After exclusion of participants with missing study variables, the analytical sample size was reduced to 1492 participants with obstructive lung function.
The 1492 participants with obstructive lung function included 907 men, 585 women, 903 whites, 315 African Americans, 232 Mexican Americans, and forty-two participants of another race or ethnicity. The mean and median ages were 55·7 and 58·0 years, respectively.
Among adults with obstructive lung function, 629 had died. The mean and median follow-up periods were 12·9 and 14·0 years, respectively. Differences in the means and percentages of study variables between participants who died and those who remained alive among adults with obstructive lung function are given in Table 1. Decedents had lower age-adjusted mean concentrations of α-carotene, β-carotene, cryptoxanthin, lutein/zeaxanthin, and total carotenoids than survivors.
Table 1 Age-adjusted baseline means and percentages of study variables among adults aged 20–79 years with obstructive lung function, by mortality status, National Health and Nutrition Examination Survey III (1988–94) (Mean values or percentages with their standard errors)

FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
In age-adjusted regression models, the concentrations of all antioxidants, except Se, modelled as continuous variables were inversely associated with all-cause mortality (Table 2). With progressive increasing levels of adjustment, the statistical significance of the hazard ratios for most antioxidants dwindled until only the concentrations of lycopene (P= 0·013) and vitamin C (P= 0·046) remained significantly and inversely associated with all-cause mortality in the maximally adjusted model (Table 2).
Table 2 Associations between antioxidant concentrations and all-cause mortality among US adults aged 20–79 years with obstructive lung function, National Health and Nutrition Examination Survey III Linked Mortality File 1988–94 to 2006 (Hazard ratios and 95 % confidence intervals)

* Estimates for each antioxidant were not adjusted for the concentrations of other antioxidants.
† Adjusted for age.
‡ Adjusted for variables in model 1 plus sex, race or ethnicity, and education.
§ Adjusted for variables in model 2 plus smoking status, alcohol consumption, leisure-time physical activity, and use of vitamin or mineral supplements.
∥ Adjusted for variables in model 3 plus systolic blood pressure, HDL-cholesterol, non-HDL-cholesterol, BMI, C-reactive protein and albumin:creatinine ratio.
¶ Adjusted for variables in model 4 plus health status, diabetes, history of myocardial infarction, and history of stroke.
The concentrations of none of the antioxidants were significantly associated with mortality from circulatory diseases (Table 3). However, the concentrations of vitamin C were inversely associated with mortality from cancer. In addition, the concentrations of lutein/zeaxanthin and lycopene were inversely associated with mortality from causes other than circulatory disease and cancer.
Table 3 Associations between antioxidant concentrations and cause-specific mortality among US adults aged 20–79 years with obstructive lung function, National Health and Nutrition Examination Survey III Linked Mortality File 1988–94 to 2006* (Adjusted hazard ratios and 95 % confidence intervals)

* Log transformations for the concentrations of β-carotene, lutein/zeaxanthin, lycopene, total carotenoids and vitamin E. Square root transformation for the concentration of vitamin C. Adjusted for age, sex, race or ethnicity, education, smoking status, alcohol consumption, leisure-time physical activity, use of vitamin or mineral supplements, systolic blood pressure, HDL-cholesterol, non-HDL-cholesterol, BMI, C-reactive protein, albumin:creatinine ratio, health status, diabetes, history of myocardial infarction and history of stroke. Estimates for each antioxidant were not adjusted for the concentrations of other antioxidants.
To examine the possibility of nonlinearity in the associations between antioxidant concentrations and all-cause mortality, a term for the squared concentrations of antioxidants was added to the proportional-hazards models, which proved to be statistically significant for lutein/zeaxanthin, total carotenoids, Se, vitamin C and vitamin E. Adjusted hazard ratios by quintiles of antioxidant concentrations are given in Table 4.
Table 4 Associations between quintiles of antioxidants and all-cause mortality among US adults aged 20–79 years with obstructive lung function, National Health and Nutrition Examination Survey III Linked Mortality File 1988–94 to 2006 (Adjusted hazard ratios and 95 % confidence intervals)
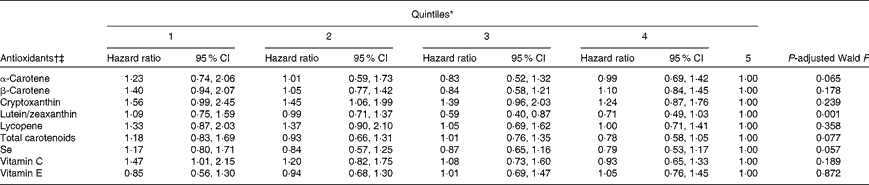
* Quintiles for α-carotene: ≤ 0·02, >0·02–0·04, >0·04–0·07, >0·07–0·11, and >0·11 μmol/l; quintiles for β-carotene: ≤ 0·13, >0·13–0·22, >0·22–0·34, >0·34–0·54, and >0·54 μmol/l; quintiles for cryptoxanthin: ≤ 0·05, >0·05–0·09, >0·09–0·13, >0·13–0·20, and >0·20 μmol/l; quintiles for lutein/zeaxanthin: ≤ 0·21, >0·21–0·30, >0·30–0·39, >0·39–0·51, and >0·51 μmol/l; quintiles for lycopene: ≤ 0·20, >0·20–0·32, >0·32–0·43, >0·43–0·56, and >0·56 μmol/l; quintiles for total carotenoids: ≤ 0·87, >0·87–1·15, >1·15–1·49, >1·49–1·97, and >1·97 μmol/l; quintiles for Se: ≤ 1·42, >1·42–1·52, >1·52–1·61, >1·61–1·75, and >1·75 nmol/l; quintiles for vitamin C: ≤ 14·76, >14·76–35·20, >35·20–49·97, >49·97–63·59, and >63·59 mmol/l; quintiles for vitamin E: ≤ 20·39, >20·39–24·68, >24·68–28·96, >28·96–35·67, and >35·67 μmol/l.
† Adjusted for age, sex, race or ethnicity, education, smoking status, alcohol consumption, leisure-time physical activity, use of vitamin or mineral supplements, systolic blood pressure, HDL-cholesterol, non-HDL-cholesterol, BMI, C-reactive protein, albumin:creatinine ratio, health status, diabetes, history of myocardial infarction and history of stroke.
‡ Estimates for each antioxidant were not adjusted for the concentrations of other antioxidants.
The associations between antioxidant concentrations and mortality did not vary by sex (P interactions >0·050). When possible interactions by race or ethnicity were examined, only the interaction term for lycopene was found to be statistically significant (Table 5). Except for β-carotene, the adjusted mean concentrations of antioxidants varied by race or ethnicity.
Table 5 Baseline adjusted mean concentrations of antioxidants associated with all-cause mortality among US adults aged 20–79 years with obstructive lung function, by race or ethnicity, National Health and Nutrition Examination Survey III Linked Mortality File 1988–94 to 2006* (Mean values with their standard errors; adjusted hazard ratios and 95 % confidence intervals)

* Log transformations for the concentrations of β-carotene, lutein/zeaxanthin, lycopene, total carotenoids and vitamin E. Square root transformation for the concentration of vitamin C. Adjusted for age, sex, education, smoking status, alcohol consumption, leisure-time physical activity, use of vitamin or mineral supplements, systolic blood pressure, HDL-cholesterol, non-HDL-cholesterol, BMI, C-reactive protein, albumin:creatinine ratio, health status, diabetes, history of myocardial infarction and history of stroke.
Detection for the presence of two-way interactions between antioxidant concentrations modelled as continuous variables and all-cause mortality was done. Significant interactions were found between the concentrations of vitamin E and those of β-carotene, cryptoxanthin, and lutein/zeaxanthin. Furthermore, significant interactions were found between the concentrations of lutein/zeaxanthin and those of cryptoxanthin and lycopene. When the concentration of each of the antioxidants was dichotomised using the median concentration and a four-level variable for each pair of antioxidants was created, none of the four-level variables was found to be a statistically significant predictor of mortality and, thus, shed little light on the interactions.
In general, antioxidant concentrations were lowest in participants who were current smokers and highest in participants who had never smoked (Table 6). Only the association between log-transformed lutein/zeaxanthin concentrations and all-cause mortality varied by smoking status (P interaction for continuous variable = 0·048). The maximally adjusted hazard ratios for continuous log-transformed concentrations were 0·62 (95 % CI 0·46, 0·83) for current smokers, 1·03 (95 % CI 0·71, 1·48) for former smokers, and 1·54 (95 % CI 0·78, 3·04) for never smokers (Table 6). The concentrations of lutein/zeaxanthin, lycopene and total carotenoids were inversely associated with all-cause mortality in current smokers.
Table 6 Baseline adjusted mean concentrations of antioxidants associated with all-cause mortality among US adults aged 20–79 years with obstructive lung function, by smoking status, National Health and Nutrition Examination Survey III Linked Mortality File 1988–94 to 2006* (Mean values with their standard errors; adjusted hazard ratios and 95 % confidence intervals)

* Log transformations for the concentrations of β-carotene, lutein/zeaxanthin, lycopene, total carotenoids and vitamin E. Square root transformation for the concentration of vitamin C. Means were back-transformed. Adjusted for age, sex, race or ethnicity, education, alcohol consumption, leisure-time physical activity, use of vitamin or mineral supplements, systolic blood pressure, HDL-cholesterol, non-HDL-cholesterol, BMI, C-reactive protein, albumin:creatinine ratio, health status, diabetes, history of myocardial infarction and history of stroke. Estimates for each antioxidant were not adjusted for the concentrations of other antioxidants.
Discussion
Oxidation is a critical factor in the pathogenesis and pathophysiology of COPD( Reference MacNee 37 – Reference Biswas, Hwang and Kirkham 39 ). Although less clear, ongoing oxidative stress may exert a deleterious effect on the prognosis of those afflicted with this condition. If so, adequate antioxidant defences might be especially important for the welfare of people with COPD. The results of the present study showing that the concentrations of vitamin C and lycopene are inversely associated with all-cause mortality in the study cohort with obstructive lung function provide measured support for this contention.
If the results of the present study do not represent chance findings, a couple of alternate explanations deserve consideration. First, vitamin C and lycopene may have in fact exerted beneficial effects in adults with obstructive lung function. Second, vitamin C and lycopene may represent risk markers for other factors that are responsible for the observed associations between the two antioxidants and mortality. Because fruits and vegetables are rich sources of both substances, one or more of the other compounds in fruits and vegetables may have been responsible for the observed associations. Along the same lines, antioxidants may represent markers for certain lifestyle characteristics that favourably affect mortality.
Prospective studies have reported that carotenoid concentrations are inversely associated with mortality( Reference Goyal, Terry and Siegel 43 – Reference Karppi, Laukkanen and Makikallio 67 ), but not all studies have done so( Reference Fletcher, Breeze and Shetty 52 , Reference Sahyoun, Jacques and Russell 68 – Reference Kataja-Tuomola, Kontto and Mannisto 70 ). These studies are supported by prospective studies of dietary or supplemental intakes of carotenoids( Reference Bates, Hamer and Mishra 64 , Reference Knekt, Reunanen and Jarvinen 71 – Reference Qiao, Dawsey and Kamangar 74 ), although other studies have failed to observe significant associations between antioxidant intake and mortality( Reference Kataja-Tuomola, Kontto and Mannisto 70 , Reference Genkinger, Platz and Hoffman 75 – Reference Roswall, Olsen and Christensen 78 ). Furthermore, in randomised trials of β-carotene, mortality was found to increase in the experimental group( Reference Bjelakovic, Nikolova and Gluud 77 , Reference Vivekananthan, Penn and Sapp 79 ). However, we were unable to find studies that have examined whether antioxidant concentrations predict mortality in adults with obstructive lung function. Therefore, the results of the present study present novel information.
Evidence regarding the association between circulating concentrations of lycopene and mortality is mixed, with several studies providing supportive evidence( Reference Ito, Kurata and Hioki 57 , Reference Ito, Wakai and Suzuki 58 , Reference Ito, Kurata and Suzuki 60 , Reference Shardell, Alley and Hicks 65 ) and others failing to do so( Reference Ito, Suzuki and Yagyu 49 , Reference Karppi, Laukkanen and Makikallio 67 , Reference Ito, Wakai and Suzuki 80 ). If lycopene reduces mortality, it is possible that COPD adults with higher lycopene concentrations would experience beneficial effects similar to those experienced by the population at large. The literature pertaining to the health effects of lycopene has zeroed in mostly on cancer( Reference Bhuvaneswari and Nagini 81 ). Of the carotenoids, the concentrations of lycopene had the strongest association with lung cancer( Reference Gallicchio, Boyd and Matanoski 82 ), a condition to which adults with COPD are particularly susceptible given their smoking history. It is possible that lycopene may exert a favourable effect on all-cause mortality by reducing the risk of lung cancer. Adults with COPD are at an increased risk of mortality from CVD( Reference Ford, Mannino and Zhao 83 ). Research has suggested that the intake and circulating concentrations of lycopene may be inversely associated with CVD( Reference Karppi, Laukkanen and Makikallio 84 – Reference Jacques, Lyass and Massaro 86 ). Besides being a strong singlet oxygen quencher( Reference Di, Kaiser and Sies 87 ), lycopene has been shown to affect endothelial function( Reference Hung, Huang and Chen 88 , Reference Armoza, Haim and Bashiri 89 ), HDL function( Reference McEneny, Wade and Young 90 ), and matrix metalloproteinase-9 induction( Reference Palozza, Simone and Catalano 91 ).
Whether the beneficial effects of adequate lycopene status specific to adults with COPD exist is less clear. Antioxidants including carotenoids, vitamin A and α-tocopherol have been demonstrated in lung tissue samples and bronchoalveolar lavage fluids( Reference Schmitz, Poor and Wellman 92 , Reference Redlich, Grauer and Van Bennekum 93 ), and supplementation with β-carotene has been shown to lead to increases in β-carotene concentrations in cells of bronchoalveolar lavage fluids( Reference Redlich, Blaner and Van Bennekum 94 ). As pointed out earlier, evidence points to inverse associations between intake and circulating concentrations of antioxidants and pulmonary function. In a trial of vegetable juice supplementation, young adults who received the vegetable juice rich in lycopene were found to experience significantly smaller reductions of FEV1 and FVC after a challenge with ozone compared with control participants( Reference Samet, Hatch and Horstman 95 ). Such potential protection could be important in adults with COPD whose respiratory reserve capacity is compromised. Lycopene has also been found to limit the inflammatory response exhibited by airway epithelial cells after a challenge with rhinovirus infection( Reference Saedisomeolia, Wood and Garg 96 ). Because acute exacerbations of COPD due to respiratory infections result in excess mortality in adults with COPD, agents that could lessen the risk of respiratory infections or limit the damage caused by respiratory infections could possibly lead to reductions in mortality. However, a great deal of study is necessary to test the effects of antioxidant supplements on morbidity and mortality among adults with obstructive lung function.
Vitamin C, which is a water-soluble essential vitamin, is involved in numerous physiological processes critical to maintaining internal homeostasis of the human body. Besides acting as a cofactor in various enzymatic reactions, it serves as an important antioxidant scavenging both reactive oxygen species and reactive N species( 97 ).
Observational prospective studies suggest that the intake and circulating concentrations of vitamin C are inversely associated with all-cause mortality or cause-specific mortality from leading chronic conditions( Reference Stahelin, Gey and Eichholzer 45 – Reference Eichholzer, Stahelin and Gey 47 , Reference Sahyoun, Jacques and Russell 68 , Reference Knekt, Reunanen and Jarvinen 71 , Reference Agudo, Cabrera and Amiano 73 , Reference Simon, Hudes and Tice 98 – Reference Jia, Aucott and McNeill 104 ). In other observational studies, however, vitamin C intake was found to have no apparent effect on mortality( Reference Fletcher, Breeze and Shetty 52 , Reference Buijsse, Feskens and Kwape 62 , Reference Genkinger, Platz and Hoffman 75 , Reference Roswall, Olsen and Christensen 78 , Reference Enstrom, Kanim and Breslow 105 – Reference Jacobs, Connell and McCullough 107 ), and a systematic review concluded that supplementation with vitamin C has no beneficial effects on mortality( Reference Bjelakovic, Nikolova and Gluud 77 ). We are not aware of studies that have examined the effect of circulating concentrations of vitamin C on mortality among adults with obstructive lung function.
The prevalence of cigarette smoking is high among adults with obstructive lung function. In the present study, about 44 % of the participants reported being current smokers. Consequently, the excess mortality among adults with COPD is in part attributable to the high prevalence of smoking. Because smoking is a prime source of oxidative stress, bolstering antioxidant defences through supplementation with antioxidants could potentially mitigate some of the ravages from smoking-induced damage to the lungs and the downstream adverse effects of oxidative stress. It has been suggested by two large trials of supplementation with β-carotene and α-tocopherol in smokers and β-carotene and retinyl palmitate in participants who had been exposed to asbestos or were heavy smokers that yielded negative consequences for the experimental groups that caution needs to be exercised while extrapolating the findings of observational studies to recommendations for using antioxidant supplementation as an approach to primary or secondary prevention( 108 , Reference Omenn, Goodman and Thornquist 109 ). The results of the present study did not show that the concentrations of β-carotene and vitamin E in current smokers were significantly associated with all-cause mortality.
The results of the present study should be viewed in the context of several limitations. A larger sample size would have provided additional statistical power to detect significant associations, to examine the associations between antioxidant concentrations and mortality in separate groups of participants with mild, moderate, and severe obstructive lung function, and to examine associations between antioxidant concentrations and cause-specific mortality. The concentrations of antioxidants were measured at a single point in time and may not have represented the usual concentrations of the study participants. Concentration data of only α-tocopherol were available in NHANES III. Interest in the health effects of γ-tocopherol has grown, and future investigations into the association between γ-tocopherol concentrations and mortality in adults with obstructive lung function would be of interest. Although a substantial number of covariates were included in the analyses, residual confounding remains a possibility as in most observational studies.
In conclusion, the concentrations of lycopene and vitamin C were inversely associated with all-cause mortality among adults with obstructive lung function. The failure of trials of β-carotene and α-tocopherol supplementation in smokers to show benefits and of antioxidant supplementation in trials of secondary prevention suggest that caution needs to be exercised before recommending antioxidant supplementation in patients with COPD. Because limited studies have examined the associations between circulating concentrations of antioxidants and all-cause mortality and cause-specific mortality in adults with COPD, additional studies in this area are needed. In addition, studies examining the intake of antioxidants from diet and supplements in adults with COPD may also yield valuable data. Lastly, trials of antioxidant supplementation in patients with COPD may be needed to fully gauge the beneficial or harmful effects of antioxidant supplementation on complications from COPD. Regardless, physicians may evaluate the nutritional status of their patients with COPD and their diets and make recommendations consistent with current dietary recommendations.
Acknowledgements
The authors’ contributions are as follows: E. S. F. conceived the study; E. S. F. and C. L. participated in the design and conduct of the study; E. S. F. conducted the analyses and drafted the manuscript; T. J. C. and J. B. C. contributed to the analysis, interpretation, and critical revision of the manuscript. All authors approved the final manuscript.
The authors declare no personal or financial interests.










