Introduction
Angiostrongylus cantonensis, the rat lungworm, is a parasitic nematode responsible for angiostrongyliasis or rat lungworm disease, and the leading cause of human eosinophilic meningitis worldwide (Hochberg et al., Reference Hochberg, Park, Blackburn, Sejvar, Gaynor, Chung, Leniek, Herwaldt and Effler2007; Graeff-Teixeira et al., Reference Graeff-Teixeira, da Silva and Yoshimura2009; Prociv and Turner, Reference Prociv and Turner2018). Infections of humans by A. cantonensis can occur through several different routes. While in many countries, angiostrongyliasis is often associated with the consumption of traditional foodstuffs, including poorly cooked or uncooked gastropods (Punyagupta, Reference Punyagupta1965; Lv et al., Reference Lv, Zhang, Liu, Zhang, Steinmann, Zhou and Utzinger2009; Eamsobhana et al., Reference Eamsobhana, Yoolek and Yong2010), in the USA, infection results primarily from the accidental ingestion of gastropods, often parts of slugs or as small neonates within uncooked plant material, i.e. vegetables and fruits, which have been inadequately cleaned (Lindo et al., Reference Lindo, Waugh, Hall, Cunningham-Myrie, Ashley, Eberhard, Sullivan, Bishop, Robinson, Holtz and Robinson2002; Slom et al., Reference Slom, Cortese, Gerber, Jones, Holtz, Lopez, Zambrano, Sufit, Sakolvaree, Chaicumpa, Herwaldt and Johnson2002; Waugh et al., Reference Waugh, Shafir, Wise, Robinson, Eberhard and Lindo2005; Cowie, Reference Cowie2013; Hollingsworth et al., Reference Hollingsworth, Howe and Jarvi2013; Yeung et al., Reference Yeung, Hayes and Cowie2013). Human infection has also been reported from drinking raw vegetable juices (Tsai et al., Reference Tsai, Lee, Huang, Yen, Chen and Liu2004, Reference Tsai, Chen and Yen2013) and home-prepared kava (a plant-based drink common in the Pacific islands) in which the semi-slug Parmarion martensi was subsequently found (Office of the Governor of Hawaii, 2017).
The potential for acquiring angiostrongyliasis from A. cantonensis third-stage larvae (L3) shed through gastropod mucus or feces is another possible route of transmission, although its epidemiological significance is unknown (Cowie, Reference Cowie2013; Kramer et al., Reference Kramer, Posner and Gosnell2017). For example, while studies using both PCR and microscopy have detected A. cantonensis in the mucus of both naturally infected P. martensi semi-slugs and experimentally infected Austropeplea lessoni snails, larvae have only been found in relatively low numbers (Qvarnstrom et al., Reference Qvarnstrom, Sullivan, Bishop, Hollingsworth and da Silva2007; Jarvi et al., Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012; Chan et al., Reference Chan, Barratt, Roberts, Lee, Shea, Marriott, Harkness, Malik, Jones, Aghazadeh, Ellis and Stark2015). Another possible source of infective larvae is from gastropod feces (personal observation) or dead and decomposing gastropods. Larvae from such sources might be disseminated throughout produce from soaking salad greens or through the reuse of contaminated water for vegetable cleaning (Crook et al., Reference Crook, Fulton and Supanwong1971; Waugh et al., Reference Waugh, Shafir, Wise, Robinson, Eberhard and Lindo2005; Howe et al., Reference Howe, Kaluna, Lozano, Torres Fischer, Tagami, McHugh and Jarvi2019). Another potential route is through the use of rainwater catchment, which is a primary source of household or agricultural water in many areas of Hawai‘i, including east Hawai‘i Island (Howe et al., Reference Howe, Kaluna, Lozano, Torres Fischer, Tagami, McHugh and Jarvi2019).
A review of the literature finds a limited number of papers that have investigated methods of rendering food safe from transmitting A. cantonensis infection. Alicata (Reference Alicata1967) investigated the use of freezing or boiling to kill A. cantonensis L3 within giant African snails (Achatina fulica) and freshwater prawns (Macrobrachium rosenbergi) and found that larvae were no longer infective when snails or prawns had been exposed to −15°C for 12 or 24 h. Similarly, he found that larvae were rendered non-infective after boiling snails for 2 or 3 min or prawns for 1 min and third-stage larvae died in water heated to between 50 and 55°C. Crook et al. (Reference Crook, Fulton and Supanwong1971) tested iodine solution (16 mg quart−1), a chemical commonly used in water treatment, against A. cantonensis L3 isolated from giant African snails (A. fulica). While larvae were reduced in number, they were not eliminated. Working with the closely related nematode Angiostrongylus costaricensis, Zanini and Graeff-Teixeira (Reference Zanini and Graeff-Teixeira2001) compared infectivity in Swiss mice after incubating isolated larvae for 1 h in wine vinegar, saturated cooking salt and a 1.5% bleach solution. Of the three treatments, 1.5% bleach showed the greatest efficacy as it killed 97% of infective larvae. Yeung et al. (Reference Yeung, Hayes and Cowie2013) also compared vinegar, bleach and salt but focused on their efficacy in removing snails and slugs from lettuce. They found none of the experimental solutions capable of removing all gastropods and none to be more effective than simply washing and rinsing with tap water.
Given the continued unmet need to reduce or eliminate rat lungworm transmission and the potential severity of angiostrongyliasis as a disease, we developed tests that allowed us to compare the efficacy of commercially available solutions and treatments in killing infectious stage (L3) A. cantonensis larvae through in vitro testing. In addition, we investigated how long isolated L3 can survive in the environment, e.g. on counters or benchtops, and under the effects of changing temperature and moisture level.
Materials and methods
Slugs were collected from multiple localities on the east side of Hawai‘i Island in either 2012 (March–October) or 2019 (May–December).
2012 Larval preparation and treatment
For tests completed in 2012, P. martensi were placed in Petri dishes where the head was removed by sterile, disposable single edge razor and an approximate 50 mg tip of the tail was collected, divided into two and put into individual DNA lysis buffer tubes (0.1M Tris HCl, 0.1M EDTA, 2% SDS) and stored at −80°C until tested by qPCR. Both head and body were minced and placed in 5 mL HCl (0.7%) – pepsin (0.5%) buffer (~2 h, 37°C) (Jarvi et al., Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012). This digestion solution was filtered twice through a modified Baermann funnel (Baermann, Reference Baermann1917; Jarvi et al., Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012). Briefly, this consists of a double-layer of KimWipes® placed in the opening of the funnel with a clamped rubber hose attached to the bottom. The solution containing nematodes was poured through the KimWipe®/funnel and allowed to sit at room temperature for at least 6 h to allow the larvae to accumulate above the clamp, and larvae were then collected.
Tubes of larvae were centrifuged at 750 rpm for 10 min, after which the supernatant was removed, and replaced with 2 mL of wash treatment of a defined dilution. The digestion buffer of HCl-pepsin was added to one microcentrifuge tube out of six to serve as a live untreated control. Tubes of larvae with treatment were then transferred to 50 mm Petri dishes, and the number of moving vs immobile larvae were visualized by ×10–×40 microscopy and recorded at 5 min intervals for a total of 20–30 min to document treatment effects on larval mobility.
2019 Larval preparation and treatment
Parmarion martensi collected for the 2019 studies were drowned in 50 mL of tap water in Falcon® tubes for a minimum of 60 h, after which slugs were removed and the bottom 15 mL from each tube was pipetted into a culture plate (60 mm × 15 mm) (Howe et al., Reference Howe, Kaluna, Lozano, Torres Fischer, Tagami, McHugh and Jarvi2019). Tap water was verified to have a chlorine content <10 ppm using Precision Chlorine Test Paper strips (Bartovation, LLC, Queens, NY, USA). Larvae exhibiting the S and Q swimming movements attributed to A. cantonensis L3 (Lv et al., Reference Lv, Zhang, Liu, Zhang, Steinmann, Zhou and Utzinger2009) were visualized by ×10–×40 microscopy (Leica S9 D and Wild Heerbrugg M4A APO) and were transferred by pipette into 35 mm × 10 mm culture plates containing approximately 2 mL of tap water. Additional washes were carried out as necessary to thoroughly separate larvae from debris.
Each treatment run included nine tubes of at least 50 larvae each: one tube of killed untreated, three tubes of live untreated, one tube of killed treated, three tubes of live treated and one tube of 50 larvae saved for later genetic analysis. Live larvae for each treatment run were prepared by adding 50 live larvae into 20 μL of tap water in three 1.5 mL microcentrifuge tubes. Live untreated larvae served as negative controls. Killed larvae served as positive controls for the propidium iodide (PI) assay and were prepared by transferring 100 larvae into 20 μL tap water, adding 500 μL of 100% methanol (BDH; VWR International, Radnor, PA, USA), followed by freezing at −80°C for a minimum of 24 h, although in most cases killed larvae were actually frozen at least 1 week (Ferreira et al., Reference Ferreira, Mendes, Bueno, de Araújo, Bartholomeu and Fujiwara2015; Jarvi et al., Reference Jarvi, Jacob, Sugihara, Leinbach, Klasner, Kaluna, Snook, Howe, Jacquier, Lange, Atkinson, Deane, Niebuhr and Siers2019). Tubes of killed larvae controls were thawed, poured into small glass Petri dishes containing 10 mL of tap water, and the methanol allowed to evaporate for 1 h with gentle rocking. Killed control larvae (n = 50) were then transferred into two 1.5 mL microcentrifuge tubes per treatment run (one treated, one untreated). A single tube of 50 larvae was transferred into DNA lysis buffer (0.1M Tris HCl, 0.1M EDTA, 2% SDS) and stored at −80°C for later genetic analysis via real-time PCR to verify the presence of A. cantonensis larvae (Jarvi et al., Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012).
One killed control tube, and three live larvae tubes were exposed to a chemical or commercial wash product at a specific concentration (including manufacturer's recommended concentration) with gentle rocking for 60 min at room temperature (unless the duration was otherwise noted, e.g. some of the salt water treatments). Following the treatment, the wash solution was removed from larvae by rinsing at least twice with 500 μL of tap water, vortexing gently, centrifuging for 5 min at 7000 rpm and removing 500 μL of the supernatant. Rinsed larvae were then transferred into culture plates containing 2 mL of tap water. Untreated larvae were vortexed and centrifuged (but not rinsed) as with the treatment washes.
Death assay: 2019
A critical difference in our determination of mortality, between 2012 and 2019, was the use of PI as a death assay in the latter year as described by Jarvi et al. (Reference Jarvi, Jacob, Sugihara, Leinbach, Klasner, Kaluna, Snook, Howe, Jacquier, Lange, Atkinson, Deane, Niebuhr and Siers2019). Thus, after treatment and wash protocols were completed, larvae were transferred into individual wells of a black, transparent-bottom view plate (#384, PerkinElmer, Waltham, MA, USA). A 1.25% solution of PI (Biotium, Freemont, CA, USA) in water was prepared for the differential staining of dead larvae (Jarvi et al., Reference Jarvi, Jacob, Sugihara, Leinbach, Klasner, Kaluna, Snook, Howe, Jacquier, Lange, Atkinson, Deane, Niebuhr and Siers2019). Twenty microlitres of PI was added to each well along with 1.2 μL of 100x penicillin-streptomycin and sufficient water to reach a final volume of 120 μL in each well. The solutions were then mixed on a 3D rotator mixer for 1 h. Plates were imaged using an Operetta high-content imaging system (Perkin Elmer Life Sciences, Boston, MA, USA) as described (Jarvi et al., Reference Jarvi, Jacob, Sugihara, Leinbach, Klasner, Kaluna, Snook, Howe, Jacquier, Lange, Atkinson, Deane, Niebuhr and Siers2019) and images collected using a ×20 long working distance lens with brightfield, PI (535 nm excitation, 617 nm emission) and fluorescein (494 nm excitation and 512 nm emission) filters. The fluorescein filter on the Operetta allowed the detection of autofluorescent live larvae, which appeared green, while under the filter for detecting PI-stain, dead larvae appeared bright yellow (Jarvi et al., Reference Jarvi, Jacob, Sugihara, Leinbach, Klasner, Kaluna, Snook, Howe, Jacquier, Lange, Atkinson, Deane, Niebuhr and Siers2019). Only larvae showing the maximum level of PI fluorescence were counted as dead. The total number of larvae and the number of larvae highly stained with PI were recorded 1 h post-staining and every 24 h for 7 days to follow the progression in L3 mortality.
Testing effects of pH, drying and temperature
We evaluated several products with various alkaline pH levels (Pinnacle M530 pH meter, Corning, Woburn, MA, USA) and compared their effectiveness in killing A. cantonensis L3 against solutions of sodium hydroxide (Cat. S318-1, Fisher Chemical, Fair Lawn, NJ, USA), buffered to pH 12.2 through 13 as described above.
Angiostrongylus cantonensis L3 were also tested for their ability to survive desiccation; either alone or after applying ethanol (Decon Labs, King of Prussia, PA, USA) or 2-propanol (Target, Minneapolis, MN, USA) as follows: 25 live and visibly moving larvae were transferred in 20 μL of water to a depression slide, then the slide water allowed to completely dry with the time recorded. When testing ethanol or 2-propanol, sufficient alcohol was added to the water on the slide to create a 70% solution, which again, was allowed to dry. Following drying, 20 μL of tap water was added to each slide according to the following times; slide 1 was allowed to dry for one additional minute before adding water, slide 2 for 2 min before adding water, and so on for up to 5 min. Within 5 min of adding water, and every 5 min thereafter, the number of both mobile and immobile larvae was counted and recorded as percentage immobility.
To test the effects of cold temperatures on larval survival, four tubes of larvae (3 live and one killed control) (n = 60 larvae/tube) were placed independently in a −15°C freezer and a 0°C refrigerator for four time periods: 0°C for 24 h; 0°C for 7 days; −15°C for 12 h; −15°C for 24 h. Refrigerator and freezer temperatures were recorded hourly for the duration of all tests using an Onset Hobo Pro v2 Humidity and Temperature Data Logger (Onset Computer Corporation, Bourne, MA, USA). After all treatments, larvae were visualized as described above and were counted every 24 h for 7 days.
Genetic analysis of larvae
Larvae were homogenized in a bead mill homogenizer as described (Niebuhr et al., Reference Niebuhr, Jarvi, Kaluna, Torres Fischer, Deane, Leinbach and Siers2020) and DNA extracted using a DNeasy® Blood & Tissue Kit (QIAGEN, Valencia, California, USA) per the manufacturer's Animal Tissue protocol with minor modification (Jarvi et al., Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012; Howe et al., Reference Howe, Kaluna, Lozano, Torres Fischer, Tagami, McHugh and Jarvi2019). Extracted DNA was analysed by real-time PCR using species-specific primers in a TaqMan Gene Expression Assay, as described in Jarvi et al. (Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012) with modification of increasing the number of cycles to 50 (Niebuhr et al., Reference Niebuhr, Jarvi, Kaluna, Torres Fischer, Deane, Leinbach and Siers2020).
Results
Gastropod collection
Due to its high rate of infection, the semi-slug P. martensi (Hollingsworth et al., Reference Hollingsworth, Kaneta, Sullivan, Bishop, Qvarnstrom, da Silva and Robinson2007; Jarvi et al., Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012; Kim et al., Reference Kim, Wong, Curry, Yeung, Hayes and Cowie2018) was the only gastropod collected as a source of A. cantonensis L3 for this study. Sources of P. martensi collected from Hawai‘i Island are provided in Table 1.
Table 1. Sources of P. martensi collected from Hawai‘i Island and drowned to obtain L3 for 2012 and 2019 trials

Study criteria
Treatment test results from both 2012 and 2019 were independently compiled, and results from replicated tests are provided as an average. The minimum concentration used to get the highest mortality is reported when multiple tests of the same treatment were repeated. Standard durations are listed as 30 min for tests done in 2012, and typically 60 min for those completed in 2019, except for salt water (sodium chloride), which was also tested for 24 and 48 h. The criteria used to measure treatment efficacy differed between the trials run in 2012 and 2019.
2012 Wash solution study
The 2012 tests used larval mobility as an indicator of potential larval mortality; therefore, efficacy was measured in terms of ‘Percent Immobility’, meaning the number of larvae that were not moving, out of the total number of larvae being evaluated (Table 2). Of the wash products tested in 2012, the majority resulted in <10% immobility including vinegar (an acid), and other common household products such as hydrogen peroxide, as well as botanical extracts, including garlic or ginger juice, oils from neem, lemongrass and peppermint. Products that did induce immobility in a high percentage of A. cantonensis included bleach (10 and 15% of the stock solution of 6% sodium hypochlorite), the vegetable wash EpiClean (Pace International, Wapato, WA, USA), the surfactant dodecylbenzene sulfonic acid (DBSA) 70% (w/w) in 2-propanol (Cat. 522953, Sigma-Aldrich, St. Louis, MO, USA) and 10% salt solution. These solutions shown to be effective in 2012 were retested in 2019 using PI differential stain as an in vitro death assay.
Table 2. Effect of treatments on larval mobility in 2012
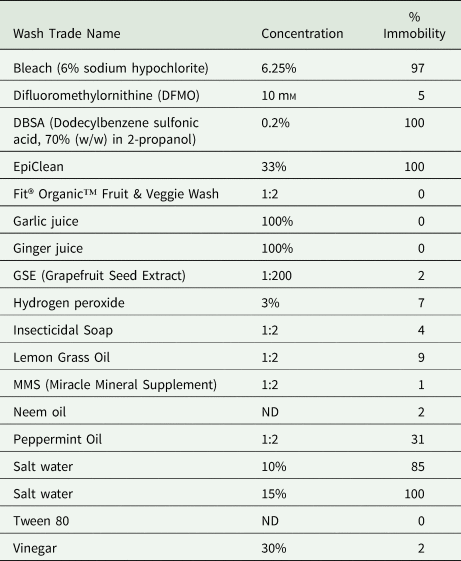
ND, not determined.
All test results from incubating L3 A. cantonensis larvae in each treatment for up to 30 min, in 5 min observation increments.
2019 Wash solution study
Efficacy in the 2019 trials is expressed in terms of ‘Percent Mortality’ as a function of the number of fluorescing larvae divided by the total number of larvae and is given for the live treated group days 0, 1 and 7 post-treatment in Tables 3 and 4. Day 0 results were collected 1 h after adding PI, Day 1 results collected 24 h post-staining, and Day 7 results illustrate the progression of mortality for 7 days. Although larvae exhibited variation in both the rate and extent of staining over the course of death, only those larvae showing PI fluorescence over the entire length of their body were counted as dead.
Table 3. Wash products showing <50% mortality rate by Day 7 using PI death assay in 2019

All test results from incubating L3 A. cantonensis larvae in each treatment for 60 min and counting fluorescent L3 1 h (Day 0), 24 h (Day 1) and 7 days (Day 7) after adding PI.
a Salt water results based upon incubation for 24 and 48 h and fluorescent L3 counted only on Day 0 (thus Day 1 and Day 7 are not applicable).
Table 4. Wash products showing >50% mortality rate by Day 7 using PI death assay in 2019

All test results from incubating L3 A. cantonensis in each treatment for 60 min and counting fluorescent L3 1 h (Day 0), 24 h (Day 1) and 7 days (Day 7) after adding PI.
a 25% dilution of the 6% stock concentration.
b Salt water results based upon incubation for 24 and 48 h and fluorescent L3 counted only on Day 0 (thus Day 1 and Day 7 are not applicable).
The results from the 2019 tests are presented in two tables: Table 3 provides results of wash products (n = 18) showing <50% kill rate by Day 7, and Table 4 provides those showing >50% efficacy by Day 7 (n = 11). Wash products showing <50% efficacy included all consumer-grade fruit and vegetable washes along with the three solutions which had appeared effective in the 2012 immobility assays: the crop wash EpiClean, the 15% salt solution for 1 h exposure and the 5% salt solution for 24 and 48 h exposure. Treatments showing more than 50% effectiveness at 1 h exposure included three commercial crop washes Field Clean, Real Clean and Sooty Mold Cleaner (Pace International), all of which were highly alkaline (pH 12.86, 12.21 and 12.45, respectively). Other effective treatments included 10 and 15% salt water for 24 h exposure, the commercial nematicide Nemitol™ (Champon Millennial Chemicals, Alexandria, VA, USA), as well as the oxidizers bleach and Keeper® Pro Post-Harvest (chlorine dioxide; Bio-Cide International, Norman, OK, USA). Surfactants, a frequent ingredient in detergents that lowers surface tension, had variable results overall, but DBSA as a 70% (w/w) solution in 2-propanol was very effective, both in terms of the speed and the thoroughness with which it killed A. cantonensis L3 nematodes.
pH, drying, alcohol and cold temperatures
pH
Because the Pace International products Field Clean, Real Clean and Sooty Mold Cleaner all demonstrated greater killing rates and had highly alkaline active ingredients, the effect of alkaline pH was tested directly on A. cantonensis L3 survival using NaOH at multiple high pH values and substantial mortality was only found at pH > 12.8 (Table 5).
Table 5. Percent mortality in L3 A. cantonensis treated with NaOH to test the effects of alkaline pH on survival using PI death assay in 2019

Drying and alcohol
Tests of L3 survival after desiccation (replicated four times) demonstrated that while an average of 85.7% of L3 could survive 1 min of drying, the number of surviving larvae dropped off sharply thereafter (Table 6). In two out of four tests, no nematodes survived longer than 3 min under desiccation conditions. Applying alcohol (70% ethanol or 2-propanol), then drying, more than doubled the number of A. cantonensis rendered immobile in the first minute compared with drying alone, although differences in mortality between all treatments were relatively minor by the third minute of drying (Table 7).
Table 6. Percent immobility of L3 after drying on hard surfaces to determine the effect of desiccation on A. cantonensis survival

Study conducted in 2019.
Table 7. Percent immobility of L3 after applying 70% alcohol, then drying on hard surfaces to determine the effect of alcohol plus desiccation on A. cantonensis survival

NA, data not available.
Study conducted in 2019.
Temperature
In comparing the survival of A. cantonensis L3 in a refrigerator (0°C) vs a freezer (–15°C), it took ~7 days at 0°C, and an entire subsequent week of observation for A. cantonensis to exhibit 99% mortality (Table 8). On the other hand, freezing L3 at −15°C for 12 h did result in death rates >50%, although there was a 2-day delay before achieving 75% mortality (Table 8).
Table 8. Mortality rates in L3 A. cantonensis from refrigeration vs freezer using PI death assay in 2019

Genetic analysis
Genetic analysis, using real-time PCR of nematodes isolated for treatment testing, showed positive results with CT values ranging 19.621–22.276, indicating the presence of A. cantonensis in all wash trials conducted in 2019.
Discussion
One of our main goals in testing a variety of wash solutions and other treatments was to compare their relative efficacy in killing A. cantonensis third-stage larvae as a means of reducing accidental infection. In 2012, we evaluated the effectiveness of wash treatments based on the degree to which they inhibited larval mobility. Use of mobility assumes that larvae that are moving must at the very least be alive, and thus potentially infective. However, lack of movement in nematodes may indicate temporary quiescence in response to environmental stress or result from sub-lethal doses of toxic or anaesthetic substances from which the larvae may eventually recover (Chandel, Reference Chandel2009; Eamsobhana et al., Reference Eamsobhana, Yoolek and Yong2010; Jarvi et al., Reference Jarvi, Jacob, Sugihara, Leinbach, Klasner, Kaluna, Snook, Howe, Jacquier, Lange, Atkinson, Deane, Niebuhr and Siers2019). In light of these concerns, in 2019, we used an alternative in vitro assay, validated in our laboratory, which uses PI stain as an indicator of L3 A. cantonensis larval death (Jarvi et al., Reference Jarvi, Jacob, Sugihara, Leinbach, Klasner, Kaluna, Snook, Howe, Jacquier, Lange, Atkinson, Deane, Niebuhr and Siers2019). In comparing our 2012 immobility and 2019 PI assays, we found that many treatments that had produced complete and long-lasting immobility in 2012 also exhibited high L3 mortality when using the PI assay. There were two exceptions: EpiClean produced 100% immobility in 2012 but, when tested with PI in 2019, showed 0% mortality. We suspect this discrepancy to be due to the significant viscosity of EpiClean, which may have inhibited larval movement. The second exception was salt water, which, when tested in 2012, produced 85% larval immobility at 10% concentration and 100% immobility at 15% (Table 2), but then <10% mortality in the 2019 PI assay (Table 3). However, this discrepancy was only found when incubating larvae in salt water for 60 min. When we exposed larvae to 10 or 15% salt solution for 24 or 48 h, we achieved 100% mortality based on the PI assay (Table 4). Therefore, our recommendations for making and using ‘slug-jugs’ (https://pharmacy.uhh.hawaii.edu/rat-lung-worm-activity-book) include leaving any potentially infected gastropods in 15% salt water for at least 24 h or longer to kill A. cantonensis escaping from their dying hosts (Howe et al., Reference Howe, Bach, DeCoito, Frias, Hatch and Jarvi2018).
Particular wash treatments were chosen for testing for a variety of reasons. Because angiostrongyliasis is both a newly emerging and potentially severe disease in Hawai‘i (Jarvi et al., Reference Jarvi, Quarta, Jacquier, Howe, Bicakci, Dasalla, Lovesy, Snook, McHugh and Niebuhr2017, Reference Jarvi, Howe and Macomber2018; Kim et al., Reference Kim, Wong, Curry, Yeung, Hayes and Cowie2018; Johnston et al., Reference Johnston, Dixon, Elm, Calimlim, Sciulli and Park2019), there are well-warranted concerns among the general population which has led to a variety of washes being recommended based on anecdotal evidence and disseminated through social media and hearsay. Many of these recommendations involve the use of common household products or ‘alternative therapies’, and we judged it important to test such claims. Another broad category of treatments in our tests includes soaps and mild detergents, generally sold to the consumer market as fruit and vegetable washes, which claim to remove bacterial and viral contamination as well as waxes and pesticide residues. Yet another category of treatments includes sprays, dips and washes used in agriculture and commercial food production. Finally, we investigated treatments that, based upon our review of the literature, showed anthelminthic potential.
Based on their mode of action, our most efficacious treatments include alkaline solutions, oxidizing agents such as bleach and chlorine dioxide, alcohols, and surfactants (compounds that lower surface tension and are frequently used in detergents). Relative to these categories, our results show general patterns. Acid solutions, such as vinegar, appear to have little effect in killing A. cantonensis L3. Given that third-stage larvae infect their rat host by passing through the acidic environment of the host stomach on to the small intestine as part of the A. cantonensis life cycle (Mackerras and Sandars, Reference Mackerras and Sandars1955; Alicata, Reference Alicata1965), such resistance to acid solutions is predictable. On the other hand, alkaline solutions, particularly when compounded with surfactants as in the case of Field Clean, Sooty Mold Cleaner and Real Clean were found to be some of the most effective treatments in killing L3-stage A. cantonensis. These three products all contain highly alkaline ingredients (potassium hydroxide in Field Clean, sodium hydroxide in the others) and measure pH 12.86, 12.45 and 12.21, respectively. In testing A. cantonensis L3 in solutions of sodium hydroxide ranging from pH 12.2 to 13, we only found substantial larvicidal effect at pH 12.8 and higher (Table 5), suggesting that the lethality of these commercial products may be due to other constituents in addition to their alkalinity.
Some of the oxidizers tested also showed considerable larvicidal capability, particularly bleach and the chlorine dioxide product Keeper® Pro Post-Harvest. Interestingly, the minimum effective concentration of household bleach in our tests was 1.5%, which is the same as that found by Zanini and Graeff-Teixeira (Reference Zanini and Graeff-Teixeira2001).
Another treatment to show promise in our tests was the insect control concentrate Nemitol™. While Nemitol™ was slow to show effect compared to other treatments, it did result in close to complete mortality by Day 7. The active ingredients of Nemitol™ – capsaicin and allyl isothiocyanate (derived from chilies and mustard oil, respectively) – are both irritants and may function as membrane disruptors (Agricultural Marketing Service, 2014; Omolo et al., Reference Omolo, Zen-Zi, Weih, Grant, Kamal and David2018; Sharma et al., Reference Sharma, Phan, Yoda, Shimokawa, Vestergaard and Takagi2019).
As a broad category, surfactants showed a variable larvicidal effect although DBSA as a 70% (w/w) solution in 2-propanol (Sigma-Aldrich) was very effective, both in terms of the speed and the thoroughness with which it killed A. cantonensis L3 nematodes. Surprisingly, treatments containing DBSA as an ingredient such as PacFoam Plus, EpiClean and EcoLab® Antimicrobial Cleaner generally showed little larvicidal capability.
Finally, our tests in both 2012 and 2019 showed very little larvicidal efficacy among consumer-grade fruit and vegetable washes as a class, nor on the part of botanical extracts such as those from ginger, garlic or lemongrass.
In addition to testing treatments as potential fruit and vegetable washes, we were interested in investigating other means of reducing infection from A. cantonensis. For example, there is considerable evidence that many gastropods shed A. cantonensis larvae when submerged in water (Cheng and Alicata, Reference Cheng and Alicata1965; Howe et al., Reference Howe, Kaluna, Lozano, Torres Fischer, Tagami, McHugh and Jarvi2019), that these larvae are capable of surviving for considerable lengths of time in water (Richards and Merritt, Reference Richards and Merritt1967; Howe et al., Reference Howe, Kaluna, Lozano, Torres Fischer, Tagami, McHugh and Jarvi2019) and that shed A. cantonensis larvae are capable of infecting rats (Crook et al., Reference Crook, Fulton and Supanwong1971). What is less well documented is the fate of larvae after the water they are in has dried. Thus, we were interested in determining whether A. cantonensis L3 would remain viable after desiccation and, if so, for how long. We found that while only an average of 14.3% of L3 were immobilized by 1 min of drying, the number of surviving larvae dropped off an additional 58%, following another minute of drying. However, the greatest variability in immobility rates also occurred at the 2 min mark; it ranged from as little as 36% to as much as 100%. These results, which showed that the majority (an average of 73%, n = 4) of L3 die after 2 min of drying agree with previous findings in the literature. Campbell and Little (Reference Campbell and Little1988) found that L3 died within minutes outside their intermediate and paratenic hosts without sufficient moisture. Richards and Merritt (Reference Richards and Merritt1967) tested the viability of third-stage larvae 2 and 10 min after allowing their surrounding water to evaporate and found that while all larvae survived for 2 min, none survived for 10 min. Thus, it appears clear that A. cantonensis L3 are unable to survive desiccation for more than a few minutes on hard, impermeable surfaces such as benchtops and doors of incubators and freezers. On the other hand, larval survival might well be extended in moist environments such as sinks and food preparation areas or possibly within snail or slug mucus.
Nevertheless, even short-term survival of L3 A. cantonensis on hands, laboratory benches and household counter-tops presents the possibility of surface-borne transmission. Therefore, we tested the effectiveness of ethanol and 2-propanol for sanitizing such surfaces. Since alcohol is typically sprayed on, then either wiped off or allowed to dry, we repeated our tests of the effect of drying but with the addition of 2-propanol or ethanol; both applied at 70% dilution. We found that the use of either alcohol more than doubled the number of A. cantonensis rendered immobile in the first minute compared with drying alone, with ethanol showing somewhat faster results. Surfaces dried before using either alcohol type would probably see even more immediate reductions in larval mobility, lending support to the utility of using alcohol in reducing the risk of transmission.
Temperature also plays a role in larval survival, with refrigeration having been previously suggested as a means of reducing infection from eating foods containing either A. cantonensis or the closely related nematode A. costaricensis (Alicata, Reference Alicata1967; Morera, Reference Morera1973; Richinitti et al., Reference Richinitti, Fonseca and Graeff-Teixeira1999). In comparing the survival of A. cantonensis L3 in a refrigerator vs a freezer, we found the effect of 0°C refrigeration on larval mortality to be ambiguous; 24 h at 0°C resulted in minimal mortality whereas 7 days at 0°C did result in steadily mounting death rates, although it took the entire subsequent week to achieve close to 100% mortality. While it is problematic to compare with Richinitti et al. (Reference Richinitti, Fonseca and Graeff-Teixeira1999), who refrigerated A. costaricensis at 5°C, it is notable that they found their L3 to remain infective after 17 days. Both of these results suggest that refrigeration by itself does not result in a sufficient death rate to serve as a reliable method of preventing Angiostrongylus infection due to ingestion of produce and undercooked food. On the other hand, freezing at −15°C, both for 12 and 24 h, clearly did result in high death rates, although again, there was a 2-day delay before achieving close to complete mortality. This agrees with the results found by Alicata (Reference Alicata1967), who demonstrated that L3 were no longer infective when exposed to −15°C for 12 or 24 h within giant African snails (A. fulica) and freshwater prawns (M. rosenbergi). One confounding observation for our L3 frozen at −15°C in the freezer was that PI results for percent mortality peaked at Day 2, then fell dramatically in the days thereafter. Because PI fluorescence is dependent upon intercalation within the bases of intact DNA (ThermoFisher Scientific, 2020), freezing and thawing are well known to have deleterious effects on DNA integrity (Srinivasan et al., Reference Srinivasan, Sedmak and Jewell2002). Given that we froze these larvae in water, that is, with no fixative such as alcohol, as was the case with the negative controls, and then stored them for 7 days at room temperature after being defrosted, we surmise that this drop-off in the percentage of PI staining to be due to decomposition of DNA from freeze–thaw.
There are inherent limitations to our study; for example, given the broad range of wash treatments tested in this study, interference and possible retardation of PI staining must be expected, and thus our measurements of mortality can only be approximations. For example, while the average mortality by day 7 post-treatment for all killed untreated was 95.2% and for live untreated 1.3%, as would be expected; day 7 average mortality for all killed treated was 87.5%, thus lower than expected. We found that many of the treatments we tested, while not capable of inducing mortality in live L3, did appear to actively degrade killed larvae, which may have resulted from the protein denaturation brought on by using methanol used to kill our nematodes. In addition, there are several other issues of a more practical nature that our study does not address; for example, the duration of time that a treatment has to be left in place to be effective in killing sufficient larvae to prevent infection may be too long for a typical consumer. As our tests were carried out in vitro, they necessarily simplify the interaction of treatments on A. cantonensis L3 mortality. Future experiments need to investigate real-world variables such as how gastropod mucus or fruit and vegetable surfaces might affect treatment efficacy. It is important that our results, particularly the more efficacious treatments, be validated in rat models (in vivo). In addition, future studies should address such questions as the extent to which our more effective treatments may affect the palatability of the fruits and vegetables with which they come in contact, and whether these treatments and the concentrations which are effective in killing A. cantonensis larvae are equally safe for human consumption. With further research into the mechanisms by which our most efficacious treatments kill nematodes, we hope to contribute towards the goal of developing a truly effective fruit and vegetable wash that would minimize risk or prevent infection by A. cantonensis.
Acknowledgements
This manuscript was written in memoriam of Dr Robbie Hollingsworth; a true scientist, a great collaborator and friend, and the motivator for this work. The authors wish to express their gratitude to the many volunteers who provided semi-slugs for our research, including Madeline Bresler, Julia DeTendresse, Alicia Giampapa, Deidre Kent and Alva Johnson and in particular, Mark Crivello and Ann Kobsa. We also wish to thank Daniel Kelly and Louis Champon for their donations of treatments, Dr Matthew Platz and Dr Hiwa Saeed for sharing their chemistry expertise and Lisa Kaluna and Kirsten A. Snook, and most particularly Cathy Mello, for their technical assistance.
Financial support
This work was supported by the Hawai‘i State Legislature, and the Daniel K. Inouye College of Pharmacy.
Disclaimer
The use of trade or corporation names within this manuscript is for the convenience of the user in identifying products. Such use does not constitute an official endorsement or approval of any product by the authors or their affiliations.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable.













