Case Summary
A 29-year-old, previously healthy woman presented to the hospital with gradual, progressive lower-extremity weakness and imbalance for a duration of 4 months. On social history, the patient endorsed chronic nitrous oxide abuse through inhalation of pressurized “whippit” canisters typically used in whipped-cream dispensers. She reported consuming in excess of 60 canisters per day (~480 g total), with the initiation of her use closely preceding the onset of her symptoms. She denied using any other recreational drugs. Motor examination of the lower extremity revealed increased tone and spasticity, an upper motor neuron pattern of weakness, increased deep tendon reflexes with spread (including crossed adductor reflex), and bilateral Babinski sign. There was a sensory level, with reduced light-touch sensation at the level of the umbilicus and loss of vibratory sensation to the level of the anterior superior iliac spine bilaterally.
Examination of her past blood-work revealed a serum vitamin B12 level that had been serially low (<75 pmol/L) for nearly 9 months. Screening blood-work at the time of presentation showed a hemoglobin level of 117 g/L, a platelet count of 243×109/L, and a white blood cell count of 4.0×109/L. Mean corpuscular volume was 90 fl. Vitamin B12 level remained less than 75 pmol/L and serum methylmalonic acid level was elevated at 3.7 µmol/L (0.05-0.27 µmol/L). Anti-parietal cell antibodies would later prove to be positive, confirming underlying pernicious anemia. The remainder of her screening blood-work was normal. The patient was admitted with a presumptive diagnosis of myelopathy secondary to B12 deficiency and was initiated on daily intramuscular B12 injections.
MRI of the spinal cord (Figures 1A-1B) performed on post-admission day 2 showed bilateral symmetric T2 hyperintensities in the dorsal columns and lateral cortical spinal tracts, in keeping with subacute combined degeneration of the spinal cord. Despite normalization of her vitamin B12 levels (696 pmol/L on post-admission day 5), the patient did not show any clinical improvement. She was discharged against medical advice on post-admission day 10 and has since been lost to follow-up.
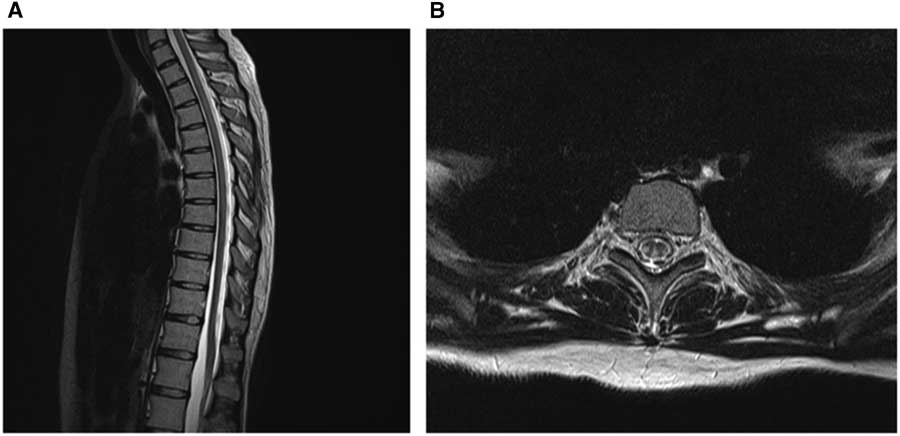
Figure 1 Nitrous oxide-induced myelopathy. Sagittal T2 MRI of the thoracic spinal cord (A) demonstrating T2 hyperintensities longitudinally through the spinal cord. Axial T2 MRI of the thoracic cord at the level of T4 (B) showing bilateral symmetric T2 signal change in the dorsal columns and lateral cortical spinal tracts.
Nitrous oxide is a commonly used inhaled anesthetic, known for its mood-elevating properties.Reference Louis-Ferdinand 1 Nitrous oxide abuse can result in myelopathy,Reference Pema, Horak and Wyatt 2 neuropathy,Reference Layzer, Fishman and Schafer 3 or myeloneuropathy.Reference Layzer 4 , Reference Vishnubhakat and Beresford 5 MRI of the spinal cord typically reveals non-enhancing T2 hyperintensity along the posterior columns and corticospinal tracts and is often longitudinally extensive.Reference Renard, Dutray, Remy, Castelnovo and Labauge 6 The mechanism by which nitrous oxide mediates neurotoxicity is thought to be by the irreversible oxidization of cobalt ions in vitamin B12, resulting in its inactivation.Reference Renard, Dutray, Remy, Castelnovo and Labauge 6 , Reference Thompson, Leite, Lunn and Bennett 7 Consequently, homocysteine conversion to methionine via methionine synthetase is impaired. Methionine is necessary in the maintenance and production of myelin, and its reduced availability leads to preferential degeneration of the myelinated posterior and lateral columns of the spinal cord. Nitrous oxide use may precipitate significant clinical deficits in patients with previously subclinical B12 deficiency, as was seen in our patient.Reference Singer, Lazaridis, Nations and Wolfe 8 Treatment of neurological complications from nitrous oxide abuse includes cessation of nitrous oxide use and vitamin B12 replacement therapy.Reference Thompson, Leite, Lunn and Bennett 7
Disclosures
The authors have nothing to disclose.
Statement of Authorship
DA performed the patient’s clinical assessment, drafted the manuscript, and created the figure. GB assisted in the patient’s clinical assessment and drafted the manuscript and figure legend. RvD reported the imaging findings, provided the figure images, and edited the manuscript. MH, ZS, and FB performed clinical assessments, provided patient care, and performed critical revisions of the manuscript for intellectual content.





