Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kuru, Y.
Marrocchelli, D.
Bishop, S. R.
Chen, D.
Yildiz, B.
and
Tuller, H. L.
2012.
Anomalous Chemical Expansion Behavior of Pr0.2Ce0.8O2-δThin Films Grown by Pulsed Laser Deposition.
Journal of The Electrochemical Society,
Vol. 159,
Issue. 11,
p.
F799.
Tuller, Harry L.
2013.
Materials for high temperature electrochemical applications: Automotive sensors, catalysts and traps.
Sensors and Actuators B: Chemical,
Vol. 187,
Issue. ,
p.
106.
Chen, Di
Bishop, Sean R.
and
Tuller, Harry L.
2013.
Non‐stoichiometry in Oxide Thin Films: A Chemical Capacitance Study of the Praseodymium‐Cerium Oxide System.
Advanced Functional Materials,
Vol. 23,
Issue. 17,
p.
2168.
Slostowski, Cédric
Marre, Samuel
Bassat, Jean-Marc
and
Aymonier, Cyril
2013.
Synthesis of cerium oxide-based nanostructures in near- and supercritical fluids.
The Journal of Supercritical Fluids,
Vol. 84,
Issue. ,
p.
89.
Kuhn, M.
Bishop, S.R.
Rupp, J.L.M.
and
Tuller, H.L.
2013.
Structural characterization and oxygen nonstoichiometry of ceria-zirconia (Ce1−xZrxO2−δ) solid solutions.
Acta Materialia,
Vol. 61,
Issue. 11,
p.
4277.
Harada, Koichiro
Oishi, Tetsuya
Hamamoto, Seiji
and
Ishihara, Tatsumi
2014.
Lattice Oxygen Activity in Pr- and La-Doped CeO2 for Low-Temperature Soot Oxidation.
The Journal of Physical Chemistry C,
Vol. 118,
Issue. 1,
p.
559.
Chen, Di
Bishop, Sean R.
and
Tuller, Harry L.
2014.
Nonstoichiometry in Oxide Thin Films Operating under Anodic Conditions: A Chemical Capacitance Study of the Praseodymium–Cerium Oxide System.
Chemistry of Materials,
Vol. 26,
Issue. 22,
p.
6622.
Andersson, Annika K. E.
Selbach, Sverre M.
Knee, Christopher S.
Grande, Tor
and
Menon, M.
2014.
Chemical Expansion Due to Hydration of Proton‐Conducting Perovskite Oxide Ceramics.
Journal of the American Ceramic Society,
Vol. 97,
Issue. 8,
p.
2654.
Kim, Jae Jin
Bishop, Sean R.
Thompson, Nicholas J.
Chen, Di
and
Tuller, Harry L.
2014.
Investigation of Nonstoichiometry in Oxide Thin Films by Simultaneous in Situ Optical Absorption and Chemical Capacitance Measurements: Pr-Doped Ceria, a Case Study.
Chemistry of Materials,
Vol. 26,
Issue. 3,
p.
1374.
Bishop, S.R.
Marrocchelli, D.
Chatzichristodoulou, C.
Perry, N.H.
Mogensen, M.B.
Tuller, H.L.
and
Wachsman, E.D.
2014.
Chemical Expansion: Implications for Electrochemical Energy Storage and Conversion Devices.
Annual Review of Materials Research,
Vol. 44,
Issue. 1,
p.
205.
Sahini, M. G.
Tolchard, J. R.
Wiik, K.
and
Grande, T.
2015.
High temperature X-ray diffraction and thermo-gravimetrical analysis of the cubic perovskite Ba0.5Sr0.5Co0.8Fe0.2O3−δ under different atmospheres.
Dalton Transactions,
Vol. 44,
Issue. 23,
p.
10875.
Chen, Yubo
Zhou, Wei
Ding, Dong
Liu, Meilin
Ciucci, Francesco
Tade, Moses
and
Shao, Zongping
2015.
Advances in Cathode Materials for Solid Oxide Fuel Cells: Complex Oxides without Alkaline Earth Metal Elements.
Advanced Energy Materials,
Vol. 5,
Issue. 18,
Swallow, Jessica G.
Kim, Jae Jin
Kabir, Mukul
Smith, James F.
Tuller, Harry L.
Bishop, Sean R.
and
Van Vliet, Krystyn J.
2016.
Operando reduction of elastic modulus in (Pr, Ce)O2− thin films.
Acta Materialia,
Vol. 105,
Issue. ,
p.
16.
Sheth, J.
Chen, D.
Kim, J. J.
Bowman, W. J.
Crozier, P. A.
Tuller, H. L.
Misture, S. T.
Zdzieszynski, S.
Sheldon, B. W.
and
Bishop, S. R.
2016.
Coupling of strain, stress, and oxygen non-stoichiometry in thin film Pr0.1Ce0.9O2−δ.
Nanoscale,
Vol. 8,
Issue. 36,
p.
16499.
Shao, Zongping
and
Tadé, Moses O.
2016.
Intermediate-Temperature Solid Oxide Fuel Cells.
p.
59.
Kim, Jae Jin
Bishop, Sean R.
Chen, Di
and
Tuller, Harry L.
2017.
Defect Chemistry of Pr Doped Ceria Thin Films Investigated by in Situ Optical and Impedance Measurements.
Chemistry of Materials,
Vol. 29,
Issue. 5,
p.
1999.
Cheng, Shiyang
Chatzichristodoulou, Christodoulos
Søgaard, Martin
Kaiser, Andreas
and
Hendriksen, Peter Vang
2017.
Ionic/Electronic Conductivity, Thermal/Chemical Expansion and Oxygen Permeation in Pr and Gd Co-Doped Ceria PrxGd0.1Ce0.9-xO1.95-δ.
Journal of The Electrochemical Society,
Vol. 164,
Issue. 13,
p.
F1354.
Zhao, Kai
and
Du, Yanhai
2017.
Calcium-doped ceria materials for anode of solid oxide fuel cells running on methane fuel.
Journal of Power Sources,
Vol. 347,
Issue. ,
p.
79.
D’Angelo, Anita M.
and
Chaffee, Alan L.
2017.
Correlations between Oxygen Uptake and Vacancy Concentration in Pr-Doped CeO2.
ACS Omega,
Vol. 2,
Issue. 6,
p.
2544.
Michel, Kathrin
Eufinger, Jens-Peter
Ulbrich, Gregor
Lerch, Martin
Janek, Juergen
and
Elm, Matthias T.
2017.
Combining two redox active rare earth elements for oxygen storage – electrical properties and defect chemistry of ceria–praseodymia single crystals.
Physical Chemistry Chemical Physics,
Vol. 19,
Issue. 27,
p.
17661.
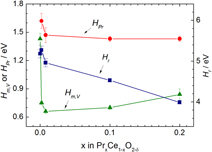
 ) regime and as such serve as model systems for investigating the correlation between thermodynamic and kinetic properties as well as exhibiting high performance figures of merit in the above applications. In this paper, we extend recently published results for Pr0.1Ce0.9O2−δ to include values of x = 0, 0.002, 0.008, 0.1, and 0.20 (in PrxCe1−xO2−δ) to test how both defect and transport parameters depend on Pr fraction. Important observed trends with increasing x include increases in oxygen ion migration energy and MIEC and reductions in vacancy formation and Pr ionization energies. The implications these changes have for potential applications of PrxCe1−xO2−δ are discussed.
) regime and as such serve as model systems for investigating the correlation between thermodynamic and kinetic properties as well as exhibiting high performance figures of merit in the above applications. In this paper, we extend recently published results for Pr0.1Ce0.9O2−δ to include values of x = 0, 0.002, 0.008, 0.1, and 0.20 (in PrxCe1−xO2−δ) to test how both defect and transport parameters depend on Pr fraction. Important observed trends with increasing x include increases in oxygen ion migration energy and MIEC and reductions in vacancy formation and Pr ionization energies. The implications these changes have for potential applications of PrxCe1−xO2−δ are discussed.



