Implications
This study explains how replacing soybean meal with yellow lupin affects duck carcass and physicochemical meat traits. The study did not show a negative effect of yellow lupin on most traits. In general, the obtained results show that the use of yellow lupin in diets for ducks may be partly a substitute for soybean meal. Soybean meal is mainly a genetically modified raw material. Consumers expect non-genetically modified products. The use of yellow lupine seeds as a protein source in duck nutrition gives broader possibilities and choice on the consumer market. The present study could support further research on the subject.
Introduction
Duck meat is popular in Asia as well as in EU countries. Pekin-type duck is popular among poultry growers and is characterised by a higher content of red muscle fibres in breast muscles compared to chicken meat, which is why duck meat is red (Graczyk et al., Reference Graczyk, Gornowicz, Mucha, Lisowski, Grajewski, Radziszewska, Pietrzak and Szwaczkowski2016; Onbasilar and Yalcin, Reference Onbasilar and Yalcin2018). Duck meat is generally regarded as flavoursome, rich in amino acids and polyunsaturated fatty acids (PUFA) and relatively low in fat. Duck meat has a high content of linoleic (C18:2 n-6) and linolenic (C18:3 n-3) acids with respect to the total content of PUFA. Cherry Valley ducks are English Pekin ducks that are reared for 8 weeks. This breed is characterised by good growth performance (Qiao et al., Reference Qiao, Huang, Chen, Chen, Zhao, Huang and Zhou2016; Cheng et al., Reference Cheng, Liu, Zhang, Zhang, Chen, Tang and Wang2017; Liu et al., Reference Liu, Li, Feng, Wang and Xia2018; Kokoszyński et al., Reference Kokoszyński, Wasilewski, Stęczny, Kotowicz, Hrnčar and Arpášová2019). Meat quality is important for producers and, above all, consumers. In addition to flavour, appearance and texture, the quality of meat is reflected in its physicochemical parameters. Meat quality traits include the colour of meat and the ability of meat to retain water, expressed by values of water-holding capacity and drip loss. Another important trait is the content of PUFA, which is beneficial to human health (Zdanowska-Sąsiadek et al., Reference Zdanowska-Sąsiadek, Michalczuk, Marcinkowska-Lesiak and Damaziak2013; Orkusz, Reference Orkusz2015). Meat quality and composition depend on genetic and environmental factors, as well as the diet of birds and the content of protein in the feed (Kuźniacka et al., Reference Kuźniacka, Adamski, Czarnecki and Banaszak2014). Soybean meal (SBM) is a popular source of protein used in poultry diets. However, alternative sources of protein are being investigated due to the expected restrictions on the use of genetically modified (GMO) SBM in animal nutrition, and local climate unsuitable for the production of soybean. Kaczmarek et al. (Reference Kaczmarek, Kasprowicz-Potocka, Hejdysz, Mikuła and Rutkowski2014) reported that protein from lupins (Lupinus spp.) is utilised to the same degree as SBM by birds. The possibility of using lupin in poultry feed depends on lupin variety and growing conditions. Old varieties of lupins were characterised by a high content of alkaloids and non-starch polysaccharides, which had a negative impact on production results and feed consumption (Rutkowski et al., Reference Rutkowski, Kaczmarek, Hejdysz and Jamroz2016). New lupin varieties used in feedstuffs are characterised by a low content of anti-nutrients (Kaczmarek et al., Reference Kaczmarek, Kasprowicz-Potocka, Hejdysz, Mikuła and Rutkowski2014 and Reference Kaczmarek, Hejdysz, Kubis, Kasprowicz-Potocka and Rutkowski2016; Hejdysz et al., Reference Hejdysz, Kaczmarek and Rutkowski2016; Rutkowski et al., Reference Rutkowski, Hejdysz, Kaczmarek, Adamski, Nowaczewski and Jamroz2017). Many studies indicate a beneficial share of yellow lupin (YL) in poultry feeding (Rutkowski et al., Reference Rutkowski, Kaczmarek, Hejdysz, Nowaczewski and Jamroz2015 and Reference Rutkowski, Kaczmarek, Hejdysz and Jamroz2016). In the research of Rutkowski et al. (Reference Rutkowski, Kaczmarek, Hejdysz and Jamroz2016), YL seeds have been shown to contain 39% of CP, while alkaloid levels have been reduced to 0.027 g/kg. Research by Kaczmarek et al. (Reference Kaczmarek, Hejdysz, Kubis, Kasprowicz-Potocka and Rutkowski2016) showed the nutritional value of different varieties of YL (Lupinus luteus L.) in broiler nutrition. The average value of CP in lupin seeds was 42.1 to 42.2 g/kg DM of seeds, which confirms the similar protein value of lupins to SBM. It should also be mentioned that lupins are a good source of essential amino acids, including lysine, compared to SBM. Lysine is an important amino acid that is often found to be deficient in birds (Koivunen et al., Reference Koivunen, Partanen, Perttilä, Palander, Tuunainen and Valaja2016). Nutrition based on lupins could improve poultry production (especially small-scale farms), because many producers are unable to produce SBM, which is caused by environmental conditions, among others (Kaczmarek etal, Reference Kaczmarek, Hejdysz, Kubis, Kasprowicz-Potocka and Rutkowski2016). Laudadio and Tufarelli (Reference Laudadio and Tufarelli2011) found no negative effect on broiler production but reported enriched PUFA profile in guinea-fowls (Tufarelli et al., Reference Tufarelli, Demauro and Laudadio2015) fed with feed based on lupins.
The aim of the study was to analyse and compare the physicochemical parameters of breast and leg muscles and fatty acid (FA) composition in breast muscles from ducks fed mixtures containing YL as an alternative to SBM. The tested hypothesis was: YL seeds used as a high-protein component as a substitute for SBM in complete feed have an effect on the quality of meat from broiler ducks.
Material and methods
Animals and diets
One-day-old Cherry Valley meat ducks were kept in pens on litter in two groups, 100 birds each. Both groups were divided into four replications with 25 birds per group. Each bird had a padlock badge for identification during measurements. The control group (1) received a balanced feed containing SBM. The treatment group (2) received a balanced feed containing YL (ground form, var. Mister). Birds were fed with 650.0 g/kg SBM (1) and 689.8 g/kg YL (2) in concentrates. The composition of feed and concentrates is presented in Table 1. The feed provided to both groups contained 55% of concentrate and 45% of wheat. Birds received feed and water ad libitum. Feed contained 140 to 190 g CP and 11.5 to 11.7 MJ metabolisable energy, consistent with recommendations by Smulikowska and Rutkowski (Reference Smulikowska and Rutkowski2018). Ducks were reared for 8 weeks with housing recommendations according to ducks. This research focused on presenting the characteristics of raw material quality (duck meat). The composition of YL seeds in nutrients was the subject of another project (yellow lupin var. Mister in the study of Kaczmarek et al., Reference Kaczmarek, Hejdysz, Kubis, Kasprowicz-Potocka and Rutkowski2016). The protein content of yellow lupin var. Mister was 40.0 g/kg DM. Productivity parameters (IBW, initial BW; BW, final BW; BWG, daily BW gain; FI, feed intake; FCR, feed conversion ratio) were controlled and calculated (Supplementary Materials S1, S2, S3) for the entire flock (100 birds per group). The research aimed at analysing meat quality. Production results were obtained from the farm where ducks were reared. After that, 16 ducks (eight from each group) of BW close to the mean for the whole group were slaughtered. Plucked and gutted carcasses were analysed in a laboratory for quality parameters.
Table 1 Composition of feed and concentrates used during 8-week rearing of ducks1

SBM = soybean meal.
1 Feed rations were established based on recommendations by Smulikowska and Rutkowski (Reference Smulikowska and Rutkowski2018): CP, 140 to 190 g/kg; metabolisable energy, 11.5 to 11.7 MJ/kg.
2 1 = feed based on soybean meal, 2 = feed based on yellow lupin.
Meat quality
The pH value of breast muscles was measured 15 min post-mortem (pH15). Carcasses were placed in cold storage at 2°C, and pH was measured again after 24 h (pH24). Both measurements were taken using a CX-701 pH meter with a knife electrode (Elmetron). Duck carcasses were weighed on RADWAG scales with accuracy to the nearest 0.01 g and then dissected using the method described by Ziołecki and Doruchowski (Reference Ziołecki and Doruchowski1989). The following parts were separated: breast muscles, leg muscles, skin with subcutaneous fat, abdominal fat, offal (liver, heart, stomach), wings with skin, neck with skin, and carcass remains (body and leg bones). Each carcass part was weighed, and dressing percentage (Supplementary Material S4) proportion in carcass was calculated (Supplementary Material S5). The colour of breast and leg muscles was assessed using a colorimeter (Konica Minolta, model CR400, Japan). The device was calibrated using white calibration plate no. 21033065 and D65Y86.1x0.3188y0.3362 scale. Colour parameters were graded according to the CIE system for L* (lightness), a* (redness) and b* (yellowness) (CIE, 1986). To measure drip loss from meat, breast muscles were weighed post-mortem (M1) and after 24-h cold storage at 2°C (M2) (Honikel, Reference Honikel1987). The water-holding capacity of breast and leg muscles was analysed (Supplementary Material S6) using a modified method from Grau and Hamm (Reference Grau and Hamm1952). Pooled samples of disintegrated muscles (about 0.300 g) were wrapped in Whatman grade-1 filter paper and kept under 2 kg pressure for 5 min. The water-holding capacity of meat was calculated (Supplementary Material S7) based on the difference in weight before and after the test (percentage of water lost from meat).
Collagen and cholesterol analysis
Collagen and cholesterol analysis in breast muscles were made according to the method described by Maiorano et al. (Reference Maiorano, Knaga, Witkowski, Cianciullo and Bednarczyk2011).
Briefly, muscle samples were lyophilised for 48 h and hydrolysed according to the methodology to determine hydroxyproline (two replicates per analysis). Then, intramuscular collagen concentration was calculated (assumption: collagen weight = 7.25 × determined hydroxyproline weight (ug/mg) of lyophilised tissue).
Cholesterol was extracted and determined by HPLC while maintaining the parameters and using equipment as described in Maiorano et al. (Reference Maiorano, Knaga, Witkowski, Cianciullo and Bednarczyk2011). Cholesterol quantification was performed according to an external standard method using a standard pure cholesterol standard (Sigma, St. Louis, MO).
Fatty acid analysis
Fatty acid composition in breast muscle was also done in our research. The used method was described by Stanek et al. (Reference Stanek, Dąbrowski, Maiorano, Tavaniello and Vizzarri2019).
Thus, according to the cited method, lipids were extracted from breast muscles and then FAs were quantified as methyl esters by gas chromatography (GC Trace 2000; ThermoQuest EC Instruments). The devices and parameters used are described in detail in Stanek et al. (Reference Stanek, Dąbrowski, Maiorano, Tavaniello and Vizzarri2019). Fatty acid peaks were identified by comparing retention time with FAMA standards (same operating conditions). The results obtained were expressed as a percentage of total identified FA. The ratio of FA n-6 to n-3 (n-6/n-3), PUFA to SFA (P/S) and atherogenic index (AI) and thrombogenicity index (TI) were calculated according to the formula of Ulbricht and Southgate (Reference Ulbricht and Southgate1991).
Statistical analysis
Numerical data were analysed using STATISTICA 10.0 PL (2011). Mean values of examined parameters and their SEM were calculated using one-way ANOVA. The significance of differences was verified with the post-hoc Scheffe test at a significance level of P-value <0.05 (Supplementary Material S8). Values of P <0.05 were statistically significant. Every chosen bird was the fundamental unit to calculate mean values in the analysis. Each bird was marked with padlock stamp. For meat quality analysis, eight birds from each group were selected (each bird had its own stamp with the number and constituted an individual unit). The calculation of production results considered the entire flock, i.e., 100 birds in each group. Standard error of mean was calculated for both groups together.
Results
Ducks’ performance
The obtained production results (Table 2) – i.e. initial and final BW, average daily weight gain, as well as feed intake and feed conversion ratio – were similar in both groups, and there were no statistically significant differences depending on the feed used (P > 0.05).
Table 2 Productivity parameters of all ducks (means, SEM) after 8-week rearing period* (n = 100 per group)

n = number of ducks (whole flock); IBW = initial BW; FBW = final BW; BWG = BW gain; FI = feed intake; FCR = feed conversion ratio (kg/kg of body gain).
1 Each value represents the mean of four replicates (25 ducks per pen).
2 1 = control group (feed based on soybean meal); 2 = experimental group (feed based on yellow lupin).
* No significant differences, P-value >0.05.
Meat quality traits
Body and carcass weight, dressing percentage as well as the weight and percentage of elements in carcass were similar between groups, and no statistically significant differences were found (P > 0.05) (Table 3). No significant differences were also found between the groups with respect to the content of muscles and fat (P > 0.05) (Table 4). Meat acidity was similar between groups in the measurements taken 15 min and 24 h post-mortem. A change of protein source in duck diet had no significant effect on pH traits (P > 0.05). Breast muscles in group 2 were characterised by a significantly higher (P < 0.05) lightness (L*) and yellowness (b*) compared to group 1. In group 2, the L* parameter was higher by 2.77 and b* by 1.79 than in group 1. The groups did not differ significantly for the colour of leg muscles (P > 0.05). The content of collagen in breast muscles was significantly higher (by 7.85 µg/mg of lyophilised muscle tissue) in group 2 than in group 1 (P < 0.05). The ability of meat to retain water, expressed by values of water-holding capacity and drip loss (percentage of water lost from meat), did not differ significantly between the groups for breast muscles (P > 0.05). For leg muscles, the water-holding capacity in group 2 was significantly higher than in group 1 (48.50% v. 37.04%; P < 0.05) (Table 5).
Table 3 Carcass traits (means, SEM) of ducks* (n = 8)

n = number of ducks used in quality analysis.
1 Each value represents the mean of four replicates (two ducks per pen).
2 1 = feed based on soybean meal, 2 = feed based on yellow lupin; values are means.
* No significant differences, P-value >0.05.
Table 4 Content of muscles and fat (means, SEM) in duck carcasses* (n = 8)

n = number of ducks used in quality analysis.
1 Each value represents the mean of four replicates (two ducks per pen).
2 1 = feed based on soybean meal, 2 = feed based on yellow lupin; values are means.
* No significant differences, P-value >0.05.
Table 5 Physicochemical parameters (means, SEM) of breast and leg muscles from ducks (n = 8)
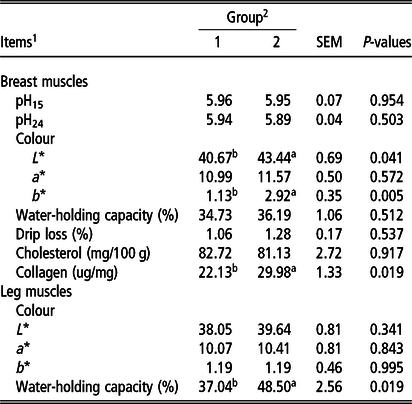
n = number of ducks used in quality analysis; pH15 = 15 min post-mortem; pH24 = 24 h post-mortem; L* = lightness; a* = redness; b* = yellowness.
1 Each value represents the mean of four replicates (two ducks per pen).
2 1 = feed based on soybean meal, 2 = feed based on yellow lupin; values are means.
a,bColumns marked with different letters differ significantly between groups, P-value <0.05.
Fatty acid composition
The total content of intramuscular fat was 1.85 g per 100 g of breast muscle in group 1 and 0.31 g lower in group 2, but the difference was not significant (P > 0.05). The analysis revealed a significantly higher content of palmitic (C16:0) and stearic (C18:0) acids, representing SFA; a higher content of arachidonic (C20:4 n-6), docosatetraenoic (C22:4 n-6) and docosapentaenoic (C22:5 n-3) acids, representing unsaturated fatty acids; and a higher total content of SFA in group 1. The total SFA content, i.e., C14:0, C16:0 and C18:0, was significantly higher by 4.56% in group 1 (P < 0.05), although the content of C14:0 did not differ significantly between the groups (P > 0.05). The content of oleic acid (C18:1 n-9), linoleic acid (C18:2 n-6) and PUFA-to-SFA ratio (P/S) were significantly higher (P < 0.05) in ducks fed YL (2: 28.94%, 23.84%, 1.08%, respectively) than in ducks fed SBM (1: 23.91%, 20.40%, 0.96%, respectively). The content of linolenic acid (C18:3 n-3) was 0.30% higher in group 2 (1.52%) than in group 1 (1.22%), but the difference was not significant (P > 0.05). The AI and TI were significantly higher in group 1 than in group 2 (P < 0.05). The content of other analysed FAs was comparable in both groups (P > 0.05) (Table 6).
Table 6 Total lipids (g/100 g) and fatty acid composition (percentage of total fatty acids) of breast muscle (means, SEM) from ducks (n = 8)

n = number of ducks used in quality analysis; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; P/S = PUFA/SFA ratio; AI = atherogenic index; TI = thrombogenic index. ΣSFA = C14:0 + C16:0 + C18:0; ΣMUFA = C16:1 n-7 + C18:1 n-9 + C20:1 n-9; ΣPUFA = C18:2 n-6 + C18:3 n-3 + C20:4 n-6 + C20:5 n-3 + C22:4 n-6 + C22:5 n-3 + C22:6 n-3; total n-6 = C18:2 n-6 + C20:4 n-6 + C22:4 n-6; total n-3 =C18:3 n-3 + C20:5 n-3 + C22:5 n-3 + C22:6 n-3; n-6/n-3 = Σn-6/Σn-3; P/S =ΣPUFA/ΣSFA; AI = [(4 × C14:0) + C16:0]/ [n-6 PUFA + n-3 PUFA + MUFA]; TI = [C14:0 + C16:0 + C18:0]/[(0.5 × MUFA) + (0.5 × n-6 PUFA) + (3 × n-3 PUFA) + n-3/n-6 PUFA].
1 Each value represents the mean of four replicates (two ducks per pen).
2 1 = feed based on soybean meal, 2 = feed based on yellow lupin; values are means.
a,bColumns marked with different letters differ significantly between groups, P-value <0.05.
Discussion
A paper by Jeroch et al. (Reference Jeroch, Kozłowski, Mikulski, Jamroz, Schöne and Zduńczyk2016) presented the results of studies investigating the effects of lupin as a protein-rich feed for poultry, including ducks, on the quality of meat. The researchers compared findings by Karasiński et al. (Reference Karasiński, Bednarczyk, Peretiakowicz and Gulewicz1988), Mihok (Reference Mihok1997), Olver (Reference Olver1997) as well as Olver and Jonker (Reference Olver and Jonker1998). Karasiński et al. (Reference Karasiński, Bednarczyk, Peretiakowicz and Gulewicz1988) reported lower weight of breast muscles from 8-week-old ducks fed a diet with a 30% inclusion of bitter lupin, compared to ducks fed sweet lupin with reduced content of alkaloids. Mihok (Reference Mihok1997) found that a 13% to 20% dietary inclusion of white lupin had no negative effect on the growth performance of Cherry Valley ducks. Olver (Reference Olver1997) as well as Olver and Jonker (Reference Olver and Jonker1998) concluded that 20% to 40% inclusion of lupin reduced the content of fat in 6-week-old ducks. In a study by Jerabek et al. (Reference Jerabek, Suchy, Strakova, Kudelkova, Simek, Jakesova, Machacek and Zapletal2018), ducks fed a diet with 50% and 100% inclusion of YL as a replacement of SBM had lower BW compared to birds fed SBM. Our study did not reveal a significant effect of dietary lupin on BW and other carcass traits of birds. Kokoszyński et al. (Reference Kokoszyński, Wasilewski, Stęczny, Kotowicz, Hrnčar and Arpášová2019) analysed the quality of duck meat from flocks of different genotypes (Pekin: P33, P8 and P9). The reported BW of 8-week-old ducks was in the range of 2417.00 to 2601.00 g. In our study, the BW of Cherry Valley ducks was about 700 g higher, and dressing percentage about 2% higher, compared to values reported by Kokoszyński et al. (Reference Kokoszyński, Wasilewski, Stęczny, Kotowicz, Hrnčar and Arpášová2019) We also found pH24 about 0.20 higher than values reported by Adamski (Reference Adamski2005) or Kokoszyński et al. (Reference Kokoszyński, Wasilewski, Stęczny, Kotowicz, Hrnčar and Arpášová2019). The decrease in pH between 15 min and 24 h post-mortem indicates correct glycolysis in breast muscles and is associated with the accumulation of lactic acid (Byrne et al., Reference Byrne, Troy and Buckley2000). In our own research, the results of pH15 and pH24 indicate correctly occurring post-mortem changes. The higher lightness (L*) and yellowness (b*) of muscles from ducks fed YL may suggest a higher concentration of carotenoids in lupin as well as a higher intramuscular fat content in muscles. Similar values of the a* parameter in both groups in our own research may indicate that duck muscles were characterised by a similar content of red muscle fibres. A higher L* value means lighter meat. This parameter can also be associated with meatʼs pH value. Zhuang and Savage (Reference Zhuang and Savage2010) found that light meat has a higher L* value at a lower pH, while dark meat has a lower L* value and a higher pH. The colour of meat can also be related to its strength, expressed by Warner-Bratzler shear force value. Light meat has a higher shear force value than dark meat. Qiao et al. (Reference Qiao, Huang, Chen, Chen, Zhao, Huang and Zhou2016) reported more than 10 units higher lightness (L*) for breast muscles compared to our study. The colour of leg muscles in both studies was comparable. Similar results were also obtained in our analyses of the ability of meat to retain water. Qiao et al. (Reference Qiao, Huang, Chen, Chen, Zhao, Huang and Zhou2016) reported the water-holding capacity of breast muscles from Cherry Valley ducks at 34.41%. In our study, the water-holding capacity was in the range of 34.73% to 36.19%. A lower value of this parameter indicates a better ability of meat to retain water (percentage of water lost from meat). According to Witak (Reference Witak2008), meat with a higher pH value has a better water retention capacity, which has been confirmed in our own research on leg muscle ability to retain water. Zhang et al. (Reference Zhang, Ah Kan Razafindrabe, Chen, Zhao, Yang, Wang, Chen, Jin and Geng2018) reported that drip loss from muscles in Chaohu ducks kept in different rearing systems was in the range of 1.09% to 1.35%, which is comparable to the values measured in our study (1.06% to 1.28%). The content of collagen, an important parameter in meat production, is an important factor in the tenderness of meat (Maiorano et al., Reference Maiorano, Filetti, Salvatori, Gambacorta, Bellitti and Oriani2001). A higher content of collagen in meat may impact meat toughness and quality (Karunaratne et al., Reference Karunaratne, Ashton and Stickland2005; Maiorano et al., Reference Maiorano, Knaga, Witkowski, Cianciullo and Bednarczyk2011). Our study revealed a higher content of collagen in breast muscles from ducks fed a diet with the inclusion of YL, which may increase meat toughness. Smith et al. (Reference Smith, Fletcher, Buhr and Beyer1993) found that collagen content was higher in the breast muscles of ducks than chickens, and that collagen growth may be age-related. Sadowska et al. (Reference Sadowska, Kołodziejska and Niecikowska2003) stated that the properties of collagen affect the higher quality of food. A lower collagen content results in greater tenderness of meat. Meat with more collagen is harder. The content of connective tissue in meat may depend on the diet of birds before slaughter (Janicki and Buzała, Reference Janicki and Buzała2013). It can, therefore, be concluded that the content of YL could have an impact on a greater amount of collagen in duck meat, which can affect the texture of meat. In turn, Baeza et al. (Reference Baeza, Dessay, Wacrenier, Marche and Listrat2002) found that muscle fibres may have a greater impact on meat texture than collagen content. It can also be concluded that ducks fed based on YL were characterised by faster growth of muscle tissue, which resulted in a higher content of collagen. Onk et al. (Reference Onk, Yalcintan, Sari, Adiguzel Isik, Yakan and Ekiz2018) analysed FA composition in 10-week-old ducks of various genotypes. They reported a lower content (under 20%) of linoleic acid (C18:2 n-6) in both treatment groups compared to our study. The content of linoleic (C18:2 n-6) and linolenic (C18:3 n-3) acids is an important element because they are essential FAs that are not synthesised by the animal (exogenous acids) – they are from a group of vitamin F. The intake of FA in the diet is of great importance for human health, including the prevention of onset and progression of cardiovascular diseases (Enser et al., Reference Enser, Hallett, Hewett, Fursey, Wood and Harrington1998; Onk et al., Reference Onk, Yalcintan, Sari, Adiguzel Isik, Yakan and Ekiz2018). Lower TI and AI indicate more beneficial effects of meat on consumer health (Ulbricht and Southgate, Reference Ulbricht and Southgate1991). The FA profile in bird meat reflects the FA content of the diet. Qiao et al. (Reference Qiao, Huang, Chen, Chen, Zhao, Huang and Zhou2016) suggest that the FA profile in feed with YL may have been different than in feed with SBM. Saturated fatty acids can have a negative impact on consumer health, while monounsaturated fatty acids (MUFA) and PUFAs are considered to have a positive effect on health. The results of FA profiles in our own work indicated that SFA was more abundant in the breast muscles of ducks based on SBM. However, the meat of ducks fed on lupin diet was characterised by a higher content of some PUFAs and MUFAs and a more desirable PUFA/SFA ratio, which indicates the partial beneficial effect of alternative feeding of ducks on meat value. Our study found lower values of TI and AI in meat from ducks fed YL, which makes it a good protein-rich feed component alternative to SBM in duck diets.
Conclusion
The replacement of SBM with YL did not cause deterioration of production results, while it improved some physicochemical parameters (lightness – L*) and the composition of some FAs, with a negative effect on collagen content in muscles and yellowness parameter (b*). Also, the composition of FA in meat from ducks fed based on YL was more balanced. Therefore, the use of YL seeds in duck diets may be recommended as a partial high-protein replacement for SBM.
Acknowledgements
The study was carried out under measure 4.4 ‘Qualitative assessment of animal raw materials produced based on domestic sources of vegetable protein’ of the multiannual programme ‘Increased use of domestic feed protein for the production of high-quality animal products under conditions of sustainable development’. This work has been supported by the Polish National Agency for Academic Exchange under grant no. PPI/APM/2019/1/00003.
M. Banaszak 0000-0002-8681-7286
J. Kuźniacka 0000-0002-0464-0326
J. Biesek 0000-0003-3050-8617
G. Maiorano 0000-0002-3387-3591
M. Adamski 0000-0001-6166-8547
Declaration of interest
The authors declare that they have no conflicts of interest.
Ethics statement
The research and duck-rearing were carried out in accordance with the local ethics committee guidelines on animal testing and care of animals. All procedures were done according to the recommendations of duck-rearing and their welfare (Poland).
Software and data repository resources
Data/models regarding the published article are not deposited in any official repository.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1751731120000610










