Background
The term “available” amino acids has widespread use across multiple research fields. Furthermore, within the nutrition field the same term conjures up numerous interpretations. The definition that the authors of this review subscribe to is that of Hurrell and Carpenter(Reference Hurrell and Carpenter1), discussed in detail by Rutherfurd and Moughan(Reference Rutherfurd and Moughan2) and put succinctly by Batterham(Reference Batterham3), whereby available amino acids refer to “the proportion of the dietary amino acids that are digested and absorbed in a form suitable for protein synthesis”, but does not refer to the amount of dietary amino acid actually utilised since utilisation is a function of both the food (dietary protein and other dietary nutrients) and the animal, while availability is a function of the dietary protein alone.
The available amino acid content of foods have been determined using growth based assays, for example the slope ratio assay(Reference Batterham, Murison and Lewis4) and more recently by the indicator amino acid oxidation method(Reference Moehn, Bertolo and Pencharz5), but these methods can only examine one amino acid at a time and strictly measure utilisation rather than availability. A simpler approach is to determine absorbed amino acids which can be achieved using the ileal digestibility assay where the absorbed amino acids are quantified from the difference between ingested amino acids and those that remain undigested at the terminal ileum after correction for endogenous ileal amino acids (true ileal digestible amino acid content). The main drawback of using true ileal digestible amino acids as a measure of available amino acids is that for some foods, particularly those that have undergone processing or cooking, the technique is not accurate for all of the amino acids. Batterham(Reference Batterham3), in a cornerstone study, showed that the ileal digestible amino acid content was an accurate predictor of the available amino acid content (determined using an animal growth assay) for a soyabean meal, but for a processed cottonseed meal, ileal digestible amino acid content overestimated the available amino acid content for lysine, threonine, methionine and tryptophan but not for isoleucine, leucine and valine. Batterham(Reference Batterham3) postulated that the observed difference between digestible and available amino acids was a result of amino acids that had been chemically modified during processing being absorbed in a form that could not be utilised. A contrary view was put forward by workers in New Zealand (the authors of this contribution), namely that the difference between available and ileal digestible amino acids instead results from inadequacies of amino acid analysis methodology. It was argued that traditional amino acid analysis methods do not accurately determine all of the amino acids, particularly those that have undergone chemical modification during processing. For the latter hypothesis to be true, the affected amino acids must be susceptible to chemical modification during processing, the modified derivatives must be either partially or completely nutritionally unavailable and the derivatives must interfere with the determination of their parent amino acids when using traditional amino acid analysis.
True ileal amino acid digestibility
To determine true ileal amino acid digestibility, a human or animal is fed a test diet and ileal digesta are collected. The amino acid content of both diets and digesta are then determined using conventional amino acid analysis and by difference digestibility is calculated. Amino acid analysis techniques have remained largely unchanged since first developed in the 1950's and involve the hydrolysis of protein in 6 M HCl at 110°C for approximately 24 h in an oxygen free environment. These hydrolysis conditions are severe and some amino acids are chemically altered during hydrolysis and cannot be quantitatively determined. For such amino acids, other hydrolytic strategies must be used. Asparagine and glutamine are determined as their acid derivatives aspartic acid and glutamic acid. Tryptophan is determined using base hydrolysis which improves tryptophan yields in comparison with hydrochloric acid hydrolysis but even then complete recovery of tryptophan is seldom achieved. For cysteine and methionine, quantitative conversion to cysteic acid and methionine sulphone using performic acid oxidation must be carried out prior to hydrochloric acid hydrolysis to improve yields, however losses of cysteine are still observed. Serine and threonine undergo hydrolytic losses such that 10–15 % of these amino acids are lost after 24 h acid hydrolysis.
Lysine
Lysine is a dietary essential (indispensible) amino acid for animals and humans. Lysine also possesses a reactive side chain amino group that can react with other compounds present in a food to form nutritionally unavailable derivatives (Maillard products)(Reference Hurrell and Carpenter1). This reaction can occur at any temperature but is greatly accelerated at elevated temperatures such as those used during heat processing or cooking. Rutherfurd and Moughan(Reference Rutherfurd and Moughan6) showed that the decrease in the reactive lysine content of heated field peas was more than three times greater after heating at 165°C compared to heating for the same length of time at 150°C. Other factors can influence the rate of Maillard product formation, including pH, water activity and reactant concentration(Reference Franzen, Singh and Okos7). Some of these lysine derivatives formed during processing or long-term storage are acid labile and can revert back to lysine during hydrochloric acid hydrolysis leading to an overestimate of lysine content(Reference Hurrell and Carpenter1). When conventional amino acid analysis techniques are employed, the reverted lysine and undamaged lysine cannot be distinguished and are determined as “total lysine” and consequently total lysine estimates may be misleading(Reference Rutherfurd and Moughan2). Furthermore, digestibility coefficients for total lysine, which are derived from the total lysine in the diet and digesta, in samples that contain significant amounts of acid labile lysine derivatives are confounded and should not be used.
A number of assays have been developed to determine reactive lysine (the lysine that possesses an unreacted side chain amino group), the most common of which include the fluorodinitrobenzene (FDNB) method(Reference Carpenter8, Reference Booth9), guanidination method(Reference Mauron and Bujard10), trinitrobenzenesulphonic acid (TNBS) method(Reference Kakade and Leiner11) and furosine method(Reference Desrosiers, Savoie and Bergeron12). However, reactive lysine is not completely absorbed from the digestive tract(Reference Moughan, Gall and Rutherfurd13) and none of the latter methods account for the incomplete digestion and absorption from the gastrointestinal tract that is common among heat-treated foods(Reference Moughan and Rutherfurd14). Moughan and Rutherfurd(Reference Moughan and Rutherfurd14) developed a method for determining the true ileal digestibility of reactive lysine whereby the reactive lysine content of both diets and digesta were determined using the guanidination reaction (reaction of O-methylisourea with the ɛ-amino group of lysine to form acid stable homoarginine) and subsequent analysis of homoarginine. The guanidination reaction with O-methylisourea (OMIU) is specific for the ɛ-amino group of lysine and OMIU does not react with the α-amino group of amino acids(Reference Moughan and Rutherfurd14–Reference Zhang, Li and Qiao21) with the exception of glycine(Reference Wang, Xu and McGrath22). Consequently, guanidination is suitable for the analysis of reactive lysine in digesta where free lysine may be present. True ileal digestible reactive lysine can then be estimated by multiplying the true ileal reactive lysine digestibility by the reactive lysine content of the food or feedstuff. From a theoretical standpoint, digestible reactive lysine is equivalent to available lysine. Moreover, Rutherfurd et al. (Reference Rutherfurd, Moughan and Morel23) conducted a study to investigate the accuracy of the assay to predict the available lysine content of a heated skim milk powder. The study, based on body lysine retention in the growing pig, employed a test diet and two control diets containing either heated skim milk powder or enzymatically hydrolysed casein (EHC) and free amino acids respectively as the sole nitrogen source. All diets were identical except for the nitrogen source and lysine in the EHC/free amino acid-based diet was assumed to be completely digested and absorbed and lysine was shown experimentally to be the first limiting amino acid in all diets. The control diets were formulated to contain lysine at the same level as the heated skim milk powder diet based on either true ileal total lysine digestibility (traditional amino acid analysis), or true ileal reactive lysine digestibility (guanidination). Rutherfurd et al. (Reference Rutherfurd, Moughan and Morel23) reported that the whole body lysine deposition of the pigs fed the heated skim milk powder-based diet was similar to that in the pigs fed the control diet formulated based on reactive lysine digestibility but was significantly and markedly different to that for the pigs fed the control diet formulated based on total lysine digestibility (Table 1). This study demonstrated the inaccuracy of total lysine digestibility and the accuracy of reactive lysine digestibility as a measure of lysine availability and showed that the inaccuracy of the true ileal digestible total lysine measure in processed feedstuffs was due to inadequacies in the traditional amino acid analytical approach rather than being due to an inherent flaw in the ileal digestibility approach.
Table 1 Least-squares means (n=8) of whole body lysine deposition (g d−1) in pigs fed a heated skim milk powder based diet and one of two EHC1 control diets

Reproduced with permission from Rutherfurd et al. (Reference Rutherfurd, Moughan and Morel23); copyright 1997 American Chemical Society.
1 Enzymatically hydrolysed casein.
2 EHC Diet A was formulated to contain lysine equal to the digestible lysine content of the heated skim milk powder determined using the conventional ileal digestibility assay (reactive lysine in heated skim milk powder x true digestibility of total lysine (determined using conventional methods) for the heated skim milk powder).
3 EHC Diet B was formulated to contain lysine equal to the digestible lysine content of the heated skim milk powder determined using the new ileal reactive lysine digestibility assay (reactive lysine in heated skim milk powder x true digestibility of reactive lysine (determined using the new method) for the heated skim milk powder).
a Means with difference superscripts were significantly (P < 0·001) different.
The difference between digestible total lysine and digestible reactive (available) lysine for processed foods and feedstuffs tested in our laboratory ranges from nil for minimally processed foods such as UHT milk or a protein supplement to 143 % for severely heat-treated feedstuffs such as moist cat food (Table 2). Consequently, ileal digestible lysine predicts available lysine accurately for some processed foods and feedstuffs but for many others the overestimation is considerable. As a result, where the accurate prediction of available lysine is required it is advisable that the digestible reactive lysine rather than digestible total lysine be determined particularly since to do so requires only an additional chemical analysis step rather than a separate bioassay.
Table 2 Digestible total and reactive (available) lysine contents (g kg−1) for a range of protein sources
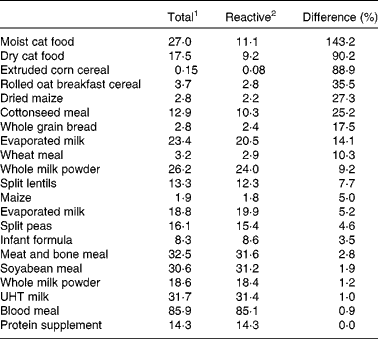
Data reported by Rutherfurd et al. (Reference Rutherfurd, Markwick-Rutherfurd and Moughan84–Reference Rutherfurd, Moughan and van Osch86) and Rutherfurd and Moughan(Reference Rutherfurd and Moughan87).
1 Based on the total lysine content of the food or feedstuff and the true ileal total lysine digestibility.
2 Based on the reactive lysine content (guanidination method) of the food or feedstuff and the true ileal reactive lysine digestibility (guanidination method).
Methionine and cysteine
The sulphur amino acids methionine and cysteine can be oxidised in foods that undergo processing or prolonged storage to methionine sulphoxide and methionine sulphone (for methionine) and cysteic acid (for cysteine)(Reference Anderson, Ashley and Jones24). In addition cysteine can also react with glucose to produce Maillard products(Reference Tai and Ho25) and with lysine to form lysinoalanine(Reference Friedman, Levin and Noma26). Methionine sulphone and cysteic acid have been shown to be nutritionally unavailable in rats(Reference Anderson, Ashley and Jones24, Reference Friedman and Gumbmann27–Reference Sjøgerg and Bostrøm29), chickens(Reference Kuzmicky, Kohler and Walker28) and turkeys(Reference Parsons and Potter30), although cysteic acid can be used by some species to synthesise taurine(Reference Anderson, Ashley and Jones24, Reference Edgar, Hickman and Marsden31). Methionine sulphoxide on the other hand can be partially utilised in rats(Reference Anderson, Ashley and Jones24, Reference Sjøgerg and Bostrøm29, Reference Baker32–Reference Njaa35), chickens(Reference Haaland, Arnesen and Njaa36), mice(Reference Friedman and Gumbmann27, Reference Baker32) and turkeys(Reference Parsons and Potter30) by the action of methionine sulphoxide reductase which converts methionine sulphoxide to methionine in vivo (Reference Moskovitz37–Reference Nakamura, Higuchi and Iwami38). The extent of dietary methionine sulphoxide utilisation is also dependent on the dietary concentrations of methionine, cysteine and methionine sulphoxide(Reference Anderson, Ashley and Jones24, Reference Haaland, Arnesen and Njaa36, Reference Gjøen and Njaa39) as dietary methionine sulphoxide will have a sparing effect on methionine so long as the amount of conversion of methionine sulphoxide to methionine required does not exceed the capacity of the methionine sulphoxide reductase enzyme.
True ileal sulphur amino acid digestibility takes into account incomplete digestion and absorption but may not give accurate values for availability for protein sources that have undergone oxidation, since the sulphur amino acids (methionine and cysteine) are traditionally determined following performic acid oxidation to quantitatively convert methionine to methionine sulphone and cysteine to cysteic acid which are then determined using amino acid analysis. Consequently, if significant amounts of methionine sulphoxide or methionine sulphone are naturally present in the food the methionine will be overestimated. The same is true for cysteic acid and cysteine. Recently, Rutherfurd and Moughan(Reference Rutherfurd and Moughan40) proposed that available methionine in oxidised foods could be predicted as shown below:
Where Metfood is the actual methionine content of the food, Met digestibility is the true ileal digestibility of methionine, MetOfood is the methionine sulphoxide content of the food, MetO digestibility is the true ileal methionine sulphoxide digestibility and MetO utilisation is the utilisation of dietary methionine sulphoxide based on the methionine, methionine sulphoxide and cysteine contents of the food. An accurate estimation of available methionine therefore requires the accurate determination of methionine and methionine sulphoxide in both diets and digesta of a test animal or human as well as an understanding of the quantitative relationship between the extent of methionine sulphoxide utilisation and the dietary concentrations of methionine, methionine sulphoxide and cysteine. To that end the analysis of methionine, cysteine and methionine sulphoxide will be discussed.
While performic acid oxidation followed by hydrochloric acid hydrolysis is the most common method used for determining sulphur amino acids in foods, in samples where all the methionine molecules are unoxidised, hydrochloric acid hydrolysis alone can be used to release methionine if rigorous removal of oxygen is undertaken prior to hydrochloric acid hydrolysis(Reference Finley41) since the loss of methionine during hydrochloric acid hydrolysis under oxygen free conditions is negligible(Reference Rutherfurd, Schneuwly and Moughan42). Methionine sulphoxide can also be reduced to methionine during hydrochloric acid hydrolysis(Reference Rutherfurd and Moughan40, Reference Puchala, Piór and von Keyserlingk43–Reference Todd, Marable and Kehrberg45) which is in contrast to the commonly held belief that methionine is oxidised during acid hydrolysis. Rutherfurd and Moughan(Reference Rutherfurd and Moughan40) suggested that this may be due to the relative flux of oxidation of methionine and reduction of methionine sulphoxide as a result of the extent to which oxygen is removed from the samples being hydrolysed. Consequently, hydrochloric acid would be an unsuitable hydrolysing agent for foods that contain significant amounts of methionine sulphoxide.
Several hydrolytic methods have been reported for determining methionine sulphoxide and these include hydrolysis with methanesulphonic acid(Reference Puchala, Piór and von Keyserlingk43–Reference Sochaski, Jenkins and Lyons44, Reference Chiou and Wang46–Reference Simpson, Neuberger and Liu47) or p-toluenesulphonic acid(Reference Hayashi and Suzuki48), and alkaline hydrolysis(Reference Sjøgerg and Bostrøm29, Reference Nakamura, Higuchi and Iwami38–Reference Gjøen and Njaa39, Reference Todd, Marable and Kehrberg45, Reference Cuq, Besancon and Chartier49). A method has also been reported based on barium hydroxide hydrolysis where methionine and methionine sulphoxide can be determined in the one analysis procedure(Reference Njaa50). Rutherfurd and Moughan(Reference Rutherfurd and Moughan40) reported that hydrolysis in methanesulphonic acid resulted in a substantial loss (50 %) of methionine sulphoxide in an oxidised fishmeal after 24 h methanesulphonic acid hydrolysis which contrasts with the findings of some workers who have reported near complete recovery of methionine sulphoxide using methanesulphonic acid hydrolysis in purified proteins(Reference Chiou and Wang46) and rumen samples spiked with pure methionine sulphoxide(Reference Puchala, Piór and von Keyserlingk43). Given the conflicting reports for the analysis of methionine sulphoxide, it would appear that more work needs to be conducted on methods for the accurate analysis of methionine sulphoxide. Furthermore, and to determine ileal methionine sulphoxide digestibility, any promising method would also need to be optimised for digesta samples.
Finally, while the discussion above describes the inadequacies of current analytical methods for determining methionine in proteins that contain methionine sulphoxide or methionine sulphone, it must also be recognised that a significant amount of methionine is used metabolically for purposes other than for protein synthesis, for example, as a methyl donor. Consequently, estimates of utilisable methionine based on body methionine retention such as the slope ratio assay will inevitably underestimate the available methionine content of food proteins as they do not account for the methionine utilised for purposes other than protein synthesis.
Threonine
During heating, threonine can react with sugars to form pyrazines, pyridines and pyrroles(Reference Ho and Chen51) with hundreds of individual compounds being identified(Reference Baltes and Bochmann52–Reference Baltes and Bochmann55). These structures, however, are unlikely to interfere with the analysis of threonine and such reactions are unlikely to be quantitatively significant under normal conditions of food processing.
The presence of phosphorylated amino acids may lead to an overestimate of the availability of those phosphorylated amino acids, particularly threonine. Phosphothreonine cannot be used directly for the synthesis of body proteins. O- and N-phospho-amino acids occur naturally in many dietary proteins but in addition, controlled phosphorylation is commonly used as a manufacturing technique to alter the functional properties of food proteins(Reference Shih56). Furthermore, uncontrolled phosphorylation may occur when foods are processed at higher temperatures.
Phosphorylated serine, threonine and tyrosine are base labile and partially acid labile(Reference Matheis, Penner and Feeney57–Reference Anderson and Kelley58). Under the hydrolytic conditions employed during amino acid analysis (6 M HCl, 24 h, 110°C) recoveries of synthetic phosphothreonine and phosphoserine have been reported to be 36 % and 2 % respectively with 46 % and 81 % of the original phospho-amino acids being dephosphorylated to threonine and serine respectively(Reference Bylund and Huang59). Similar results were observed for both phosphothreonine and phosphoserine when hydrolysed from phosphothreonine and phosphoserine containing peptides(Reference Bylund and Huang59). It is likely that for processed protein sources conventional hydrochloric acid hydrolysis overestimates the amounts of threonine and serine if the phosphorylated forms of these amino acids are present and this may have implication for the determination of available threonine and serine if phosphothreonine and phosphoserine cannot be utilised. In addition, as is the case for unphosphorylated threonine and serine, not all the phosphothreonine and phosphoserine can be recovered during hydrochloric acid hydrolysis.
Threonine is a quantitatively important amino acid in the production of intestinal proteins, particularly mucins. As much as 60-100 % of the absorbed threonine is extracted and utilised by the portal drained viscera (PDV) (the intestine, pancreas, spleen and stomach) of pigs(Reference Stoll, Henry and Reeds60–Reference van der Schoor, Reeds and Stoll61). Furthermore, systemic amino acids would appear to be poorly utilised by the intestine since Dudley et al. (Reference Dudley, Wykes and Dudley62) showed that mucosal protein synthesis was substantially lower in piglets fed parenterally compared to those fed enterally. Thus the bulk of dietary threonine is used for PDV protein synthesis and the bulk of the threonine used for PDV protein synthesis is likely to be of dietary origin.
Alkaline phosphatase catalyses the dephosphorylation of a wide range of molecules and is present in the enterocytes of the small intestine, predominantly in the duodenum(Reference Hietanen63–Reference Nakano, Kawamoto and Takano65). However, during a meal containing large amounts of phosphothreonine the activity of intestinal alkaline phosphatase may be insufficient to quantitatively dephosphorylate the absorbed phosphothreonine before it is transported from the brush border to the portal vein. Consequently, the latter phosphothreonine molecules would be largely lost to PDV protein synthesis. The absorbed phosphothreonine may be dephosphorylated in the body but given that most of the threonine requirement is for intestinal protein synthesis the net result may be poor utilisation of threonine in processed foods that contain large quantities of phosphothreonine.
The impact of phosphorylation on the in vitro digestibility of phosphorylated casein(Reference Matheis, Penner and Feeney57), and soya bean proteins(Reference Hirotsuka, Taniguchi and Narita66–Reference Sung, Chen and Liu67) using a selection of proteases has been reported to be minor. In contrast, Giec et al. (Reference Giec, Stasifiska and Skupin68) reported a 10 % decrease in the in vitro digestibility of a yeast homogenate after phosphorylation. To the authors' knowledge no in vivo studies have been conducted investigating the impact of phosphorylation on protein digestibility. Matheis et al. (Reference Matheis, Penner and Feeney57) used a tetrahymena bioassay to show that a broth containing phosphorylated casein reduced the growth of T. thermophili by 20 % in comparison to a broth containing proteose peptone (animal protein hydrolysate) suggesting that with T. thermophili, at least, there was a lower utilisation of phosphorylated amino acids compared to their unphosphorylated counterparts.
To summarise, significant amounts of phosphothreonine may be present in some processed food proteins. The presence of phosphothreonine probably does not significantly affect protein digestibility but may impact on threonine utilisation if the phosphorylated threonine cannot be dephosphorylated in the brush border at a rate sufficient to support PDV protein synthesis.
Tryptophan
Tryptophan can also be modified during processing, usually through oxidation, to a range of products depending on the oxidising agents present(Reference Simat and Steinhart69). Weck et al. (Reference Weck, Nielsen and Finot70) have shown that the tryptophan oxidation products generated after hydrogen peroxide treatment are not nutritionally available. Tryptophan can be quantitatively recovered during the hydrochloric acid hydrolysis of pure proteins(Reference Gruen and Nicholls71), but in foods where significant amounts of carbohydrates are present tryptophan recoveries tend to be low(Reference Finley41). Consequently, base hydrolysis is commonly used for the determination of tryptophan in foods but even then recoveries are incomplete(Reference Finley41).
When analysing samples for tryptophan content, it is common practice to include an internal standard, usually 5-methyl tryptophan, and to correct the determined tryptophan values based on the recovery of the internal standard. This method relies on a similar loss rate for both tryptophan and the internal standard. If the loss rate of the internal standard during hydrolysis is greater than that of tryptophan, then tryptophan content will be overestimated. A disparity in loss rates between tryptophan and the internal standard may explain why ileal digestible tryptophan overestimates available tryptophan in some proteins. However, it is the experience in our laboratory that the loss rates of tryptophan and 5-methyl tryptophan are similar and if they do differ 5-methyl tryptophan degrades more slowly than tryptophan which would lead to an underestimate of tryptophan rather than an overestimate. Overall, more work is required for the accurate analysis of tryptophan in foods and digesta.
Least-squares nonlinear regression
While many amino acids are stable under the strong acidic conditions used to hydrolyse proteins for amino acid analysis, some amino acids, for example cysteine, methionine, serine, threonine and tryptophan, are destroyed either partially or totally during hydrochloric acid hydrolysis. Typically hydrolytic losses of 10–15 % for serine and 5–10 % for threonine are not uncommon after 24 h hydrolysis(Reference Darragh, Garrick and Moughan72–Reference Ozols73). In contrast, branched chain amino acids are difficult to hydrolyse and require more than 24 h hydrolysis for complete liberation. Essentially, the hydrolysis interval of 24 h is a compromise that permits the quantitative hydrolysis and recovery of most but not all amino acids. Hydrolysis for 72–96 h has been used to determine isoleucine and valine content(Reference Rayner74–Reference Rowan, Moughan and Wilson75), while using multiple hydrolysis intervals and extrapolating (linear regression) back to 0 h hydrolysis has been used to estimate serine and threonine content(Reference Rowan, Moughan and Wilson75). The latter approach assumes that the release of an amino acid from the protein and its subsequent degradation occurs sequentially but it is much more likely that for any given amino acid, liberated residues begin to degrade while other residues are yet to be hydrolysed. Least-squares nonlinear regression, which takes into account the simultaneous hydrolysis and destruction of amino acids has been used to estimate the amino acid contents of foods and other protein sources(Reference Rutherfurd, Schneuwly and Moughan42, Reference Darragh, Garrick and Moughan72, Reference Darragh and Moughan76–Reference Rutherfurd80). The method involves determining the amino acid yield for samples that have been hydrolysed over multiple intervals and using least-squares nonlinear regression to estimate the amino acid content based on the following model:
Where A o is the protein bound amino acid content, B(t) is the amino acid concentration at time t, Bo is the free amino acid concentration prior to hydrolysis, h is the hydrolysis rate (the rate at which amino acids are released from the protein), l is the loss rate (the rate at which amino acids are degraded).
In Table 3 the threonine and serine contents predicted using either least-squares nonlinear regression or determined after conventional 24 h hydrochloric acid hydrolysis are given for several protein sources. The threonine and serine content determined using 24 h hydrochloric acid hydrolysis underestimated that predicted using least-squares nonlinear regression by 3–13 % for threonine and 2–14 % for serine. The main drawback of the least-squares nonlinear regression is the time and cost to run the analyses, given that multiple (between 10 and 25) hydrolysis intervals are required. Consequently, this method is not suitable for routine amino acid analysis of foods. Where it does have a place, however, is when highly accurate amino acid compositional data are required.
Table 3 Threonine and serine contents (g kg−1) of selected protein sources predicted using either least-squares nonlinear regression or determined after conventional 24 h hydrochloric acid hydrolysis
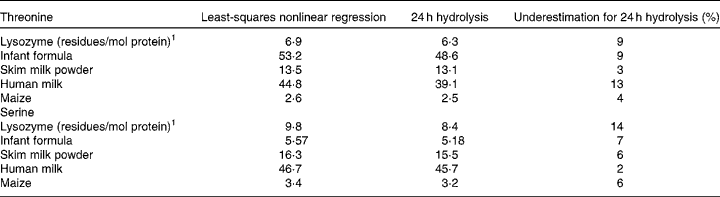
Data reported by Rutherfurd et al. (Reference Rutherfurd, Moughan and Lowry79), Rutherfurd(Reference Rutherfurd80) and Darragh and Moughan(Reference Darragh and Moughan76) and Darragh et al. (Reference Darragh, Garrick and Moughan72).
1 The actual threonine and serine content of lysozyme based on amino acid sequence is 7 and 10 residues/mol protein(Reference Canfield88).
Racemisation
Racemisation is another effect of processing, particularly at high pH, leading to the formation of D-amino acids. Amino acids will racemise at differing rates depending on the electron-withdrawing ability of the amino acids side chain(Reference Liardon and Ledermann81) with amino acids like cysteine, serine and threonine being the most affected, and the neutral amino acid valine, isoleucine and leucine being the least affected(Reference Liardon and Ledermann81). The proteins within which the amino acids are bound also impact on amino acid racemisation rates(Reference Liardon and Ledermann81). Negligible racemisation occurs in heat-treated proteins in the absence of alkaline treatment after heating at moderate temperatures (120°C for 1 h), and low levels ( < 3 %) of racemisation occurs for most amino acids after heating at high temperatures (230°C for 20 min)(Reference Liardon and Hurrell82). D-amino acids are generally not nutritionally available but conventional amino acid analysis determines the D- and L-amino acids collectively thereby overestimating the available amino acid content in alkaline-treated proteins. For proteins that contain significant amounts of the D-amino acid enantiomers, analytical methods that can distinguish between D- and L-amino acids are required. Such methods are discussed in detail by Rutherfurd and Sarwar-Gilani(Reference Rutherfurd and Sarwar-Gilani83).
Conclusions
The available amino acid content of a food is the amount of dietary amino acids absorbed from the gut in a chemical form that can be used for protein synthesis. True ileal digestible amino acid content is a good predictor of available amino acid content for unprocessed foods and for most amino acids in processed foods. However, for some amino acids, mainly lysine, methionine, cysteine, threonine and tryptophan ileal digestible amino acid content can be a poor predictor of the available content. The differences, by and large, relate to inadequacies of current analytical methodologies to accurately determine the amino acid content in foods and digesta that contain amino acids modified during processing. For these amino acids, other analytical strategies must be adopted if available amino acids are to be determined accurately.
Acknowledgements
SR wrote the manuscript and PM reviewed and edited the manuscript. The authors state that there are no conflicts of interest. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.





