Substantial epidemiological data indicate that low serum vitamin D concentrations, or surrogates for vitamin D status, such as geographical latitude or season, are associated with an increased risk of a variety of cancers in humans(Reference McCullough, Weinstein and Freedman1–Reference Yin, Grandi and Raum7). Evidence is strongest and most consistent for colorectal carcinoma and breast cancer(Reference Ng, Meyerhardt and Wu4–Reference Yin, Grandi and Raum7). The mechanisms by which vitamin D status can alter cancer development are still being studied, but what is now known can be summarised briefly. Many cell types contain vitamin D receptors(Reference Deeb, Trump and Johnson8) and when these receptors are activated by 1,25-dihydroxycholecalciferol, the most active metabolite of vitamin D, they induce differentiation and inhibit proliferation, invasiveness, angiogenesis and metastatic potential(Reference Deeb, Trump and Johnson8). When vitamin D status is suboptimal, these activities may be impaired. For example, mice rendered vitamin D deficient exhibit enhanced cancer development and cancer growth, as do vitamin D receptor knockout mice(Reference Welsh9). In addition, the vitamin D cancer hypothesis has been supported by laboratory studies that show that 1,25-dihydroxycholecalciferol has potent antineoplastic activity against numerous tumour cells(Reference Rassnick, Muindi and Johnson10). In dogs, however, unlike man, serum vitamin D is not influenced by UV exposure, since dogs do not convert 7-hydroxycholesterol into vitamin D3, due to very low concentrations of 7-hydroxycholesterol(Reference How, Hazewinkel and Mol11). This difference in environmental exposure leaves dietary intake as the primary means of obtaining vitamin D2 or vitamin D3 for conversion to 25-hydroxyvitamin D3 (25(OH)D3), thereby eliminating a significant variable during investigation.
Cutaneous mast cell tumours (MCT) occur more frequently in dogs than in any other species; MCT account for 7–21 % of all canine skin and subcutaneous tumours(Reference Dorn, Taylor and Schneider12, Reference Cohen, Reif and Brodey13). MCT usually develop in both male and female older-aged dogs. Although MCT have been reported in most breeds, the risk is increased in Labrador retrievers; in two combined studies of dogs with MCT, approximately 25 % were Labrador retrievers(Reference Rassnick, Moore and Williams14, Reference Rassnick, Bailey and Flory15). The increased prevalence of MCT in certain breeds, such as Labrador retrievers, suggests an underlying genetic cause; to date, defects predisposing dogs to MCT have not been elucidated. The majority of canine MCT express the vitamin D receptor, a likely prerequisite to respond to vitamin D or its derivatives(Reference Russell, Rassnick and Erb16). Furthermore, our laboratory group has shown that, in vitro, vitamin D metabolites have anti-proliferative effects on the growth of canine mastocytoma cells. Additionally, high oral dosing of 1,25-dihydroxycholecalciferol induces tumour regression in dogs with spontaneously occurring MCT, yet not without clinical signs of toxicity(Reference Malone, Rassnick and Wakshlag17). Taken together, these data suggest a possible role for vitamin D in the genesis and/or course of MCT in dogs. The objective of the present cross-sectional study was to investigate 25(OH)D3 serum concentrations as an indicator of an individual's vitamin D intake(Reference Deeb, Trump and Johnson8), in Labrador retrievers with MCT.
Experimental methods
Study groups
Client-owned Labrador retrievers with cutaneous MCT were included as affected cases. Histology or cytology of the cutaneous MCT was sufficient for inclusion as a case, with all samples acquired before surgical or medical intervention. Healthy, client-owned Labrador retrievers > 7 years of age served as non-affected controls and were enrolled on the basis of a normal medical history and physical examination performed by an oncologist and veterinary technician. For both cases and controls, dogs < 2 years of age or with known hepatic insufficiency, renal insufficiency, diabetes or clinically significant systemic or infectious diseases were excluded. Dog-owners provided written informed consent to the study, which was approved by the Institutional Animal Care and Use Committee at Cornell University.
Study variables
Blood and urine were sampled from each subject after fasting for ≥ 8 h. Blood samples were protected from light, allowed to clot, and then centrifuged at 3800 g for 10 min. A sample of serum was immediately stored at − 70°C until analysis of 25(OH)D3 was done. 25(OH)D3 levels were measured at the Michigan State College of Veterinary Medicine using a validated RIA kit. Serum chemistry and urinalysis were performed on remaining samples of serum and urine to ensure adequate organ function. At enrolment, all dogs were recorded as having either single, multiple or gross (metastatic) mast cell disease, surgical removal or not, and tumour grade when available. Dog owners completed a questionnaire that included questions about dietary intake during the 3 months before enrolment. Information regarding the cholecalciferol and ergocalciferol content of dog foods, treats and supplements was acquired by contacting manufacturers. Dietary vitamin D intake was calculated to represent μg intake per d based on portion sizes of foods, treats, table foods and supplements fed. Dietary vitamin D intake was then calculated by dividing the daily vitamin D intake by body weight in kg. If the dog was fed ≥ 25 % table foods, or was given fish, dairy foods, liver or other by-products, or animal product oils, those owners were contacted for a more complete diet history. The US Department of Agriculture database for vitamin D concentrations in commonly eaten foods was used to assess the vitamin D content of table foods given. The following information about each case and control was recorded: body condition score (BCS; on a scale of 1 to 5, where, briefly, a BCS of 1 was consistent with emaciation; BCS of 2 was thin; BCS of 3 was optimal; BCS of 4 was overweight; BCS of 5 was obese), body weight, sex, age and μg vitamin D intake per kg body weight.
Statistics
Analysis of the data was performed using non-paired Student's t tests for the examination of serum 25(OH)D3 and calculated intake between affected and control Labradors retrievers due to normally distributed data (SAS 9.0; SAS Institute, Inc., Cary, NC, USA). To examine the influence of age and BCS, the data were dichotomised for BCS in affected and control Labradors as < 4 or ≥ 4, and age was dichotomised into ≤ 10 years old or > 10 years old for multivariable regression statistics (SAS 9.0; SAS Institute, Inc.). Significance for all variables and testing was set at P = 0·05.
Results
Between June 2009 and September 2010, eighty-seven Labradors retrievers were enrolled, including thirty-three cases with cutaneous MCT, and fifty-four unaffected controls (Table 1). Of the affected dogs, twenty-six of twenty-eight examined histologically were grade 2 tumours, with five dogs being diagnosed through cytological examination. Of the thirty-three affected dogs, eleven had surgical excision of their tumours before oncology consultation and blood collection for the present study (Table 1). The mean serum 25(OH)D3 concentration for all dogs was 114 (sd 34) nmol/l. Mean serum 25(OH)D3 concentration in Labrador retrievers with MCT (104 (sd 30) nmol/l) was significantly lower than that of unaffected Labrador retrievers (120 (sd 35) nmol/l; P = 0·027; Fig. 1). The multivariable analysis, after controlling for age ( ≤ 10 and > 10 years) and BCS ( < 4 or ≥ 4), showed that serum 25(OH)D3 was still significantly lower in affected dogs (P = 0·022). Age showed a trend toward significance (P = 0·052) and BCS was not significant (P = 0·651) in the model. Due to the trend towards significance of dichotomised age in the generalised linear model, a linear regression analysis of age v. serum 25(OH)D3 was performed; Pearson's product moment was not significant (R 0·15; P = 0·23). The mean calculated vitamin D intake per kg body weight in affected Labrador retrievers was extremely variable, and therefore not statistically different from that of unaffected Labrador retrievers (0·38 (sd 0·25) and 0·31 (sd 0·22) μg/kg body weight, respectively; P = 0·13).
Table 1 Characteristics of Labrador retrievers in the case–control cross-sectional study to evaluate serum 25-hydroxyvitamin D3 as a risk factor for cutaneous mast cell tumours (MCT)
(Mean values and standard deviations or number of animals)
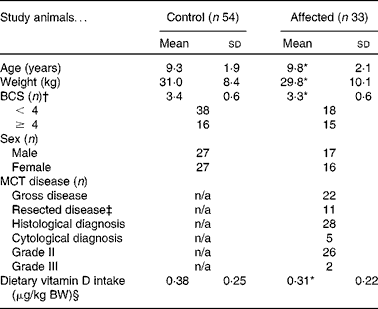
BCS, body condition score; n/a, not applicable; BW, body weight.
* There was no statistically significant difference between the affected dogs and the unaffected control dogs.
† BCS of 1 was consistent with emaciation; BCS of 2 was thin; BCS of 3 was optimal; BCS of 4 was fat; BCS of 5 was obese.
‡ Median time from surgery was 13 d.
§ Calculated by multiplying the daily frequency of each consumed food item by the nutrient value of the portion size (see Experimental design section). Manufacturers were contacted for dietary information for twenty-five MCT cases and forty-two controls.

Fig. 1 Box and whisker plot showing serum 25-hydroxyvitamin D3 (25(OH)D3) in affected (n 33) and control (n 54) Labrador retrievers. Values are means (—), with standard deviations (boxes) and the 75th and 25th interquartile ranges represented by vertical bars; outliers (○) are depicted. * Mean value was significantly different from that of the control dogs (P = 0·027; Student's t test).
Discussion
The present results indicate that among Labrador retrievers, serum 25(OH)D3 concentrations were significantly lower in dogs with cutaneous MCT compared with unaffected controls, suggesting that low serum vitamin D might be a risk factor for the development of this tumour in this breed. As a cross-sectional study, the association between a particular exposure and outcome is difficult to interpret; therefore the causal relationship or risk factor for developing MCT in Labrador retrievers with low serum vitamin D cannot be determined. Cohort studies evaluating serum vitamin D status as a risk factor for MCT development over an extended time period are warranted.
Most 25(OH)D3 serum concentrations in dogs in both groups were within the reference range (60–215 nmol/l) used by the diagnostic laboratory in which it was tested. To our knowledge, this reference range is based on the standard curve of a population of healthy dogs. Subclassification of vitamin D status into ‘deficiency’, ‘insufficiency’, or ‘adequate’, as done in humans(Reference Deeb, Trump and Johnson8), has not been done in dogs. The threshold of the serum 25(OH)D3 that separates vitamin D sufficiency from insufficiency might be able to be defined from biological effects such as those on Ca homeostasis or skeletal metabolism. Others have shown that the threshold of serum 25(OH)D3 levels that induce an increase in serum parathyroid hormone is in important measure of vitamin D adequacy(Reference Chapuy, Preziosi and Maamer18, Reference Krall, Sahyoun and Tannenbaum19). Such an investigation might prove useful in dogs.
There was a mild, yet insignificant, negative correlation between age and serum 25(OH)D3 concentration. Evaluation of a larger sample size might lead to a significant correlation. The true importance of this relationship would be questionable, however, since age is a strong predictor of cancer, incorporating the cumulative effect of many exposures and events. Additionally, there was no association between BCS and vitamin D status in this population of dogs. In humans BMI is inversely correlated with serum vitamin D concentrations; higher BMI or obesity has been found to be associated with substantially lower serum vitamin D concentrations, possibly due to decreased bioavailability from sequestration of vitamin D in body fat, differences in sun exposure among populations, or negative feedback from higher circulating 1,25-dihydroxyvitamin D(Reference McCullough, Weinstein and Freedman1, Reference Giovannucci20).
There was no significant difference in the mean vitamin D intake between dogs affected with MCT and the control population. Dogs, unlike man, have no ability to synthesise vitamin D from cholesterol precursors and UV irradiation at the level of the skin, making oral intake of either cholecalciferol or ergocalciferol a dietary requirement(Reference How, Hazewinkel and Mol11). As such, commercial pet foods are routinely supplemented with these ingredients. Our calculations for vitamin D intake might not be accurate, since many pet food companies do not have their products analysed for vitamin D content and only provide the information based on additional vitamin D (cholecalciferol) added to the food. Overall intake of vitamin D can vary widely in dogs depending on the amounts of calcitriol, cholecalciferol or ergocalciferol in the products used in the manufacturing of pet foods which are not necessarily accounted for in some diets, particularly when large amounts of fish, dairy produce or by-products are used (personal communication – Dr J. Morris; University of California, Davis, CA, USA). If vitamin D intake was in fact similar between both groups of dogs, this would suggest that other factors involved in vitamin D metabolism, such as cytochrome p450 enzymic activity, might be responsible for differing serum 25(OH)D3 concentrations.
The increased incidence of MCT in certain breeds, such as Labrador retrievers, suggests an underlying genetic cause. However, it is possible that the aetiology of MCT is a combination of genetic factors acting in response to environmental or external factors. The compelling biological links between vitamin D and cancer in humans has motivated the search for such a link in dogs. The present study showed that vitamin D concentrations, measured by 25(OH)D3, were lower in Labrador retrievers with MCT compared with unaffected dogs of the same breed. Future studies should investigate possible explanations for the significant differences between the groups and evaluate vitamin D status as a predictor for the subsequent development of MCT.
Acknowledgements
The present study was supported by a grant from Nestlé Purina. The authors thank Drs Renee Al-Sarraf, Mike Kiselow, Tim Rocha, John Chretin, Brenda Phillips and Courtney Zwahlen for contributing samples to the present study, as well as Dr Marta Castelhano and Dr Rory Todhunter for providing samples from normal healthy Labrador retrievers as part of their ongoing National Institutes of Health (NIH) R24 grant no. 1R24GM082910-01A1.
All data were kept confidential and participants' data were kept anonymous. Compliance with codes of ethics for research and publication were maintained.
All authors contributed significantly to the work.
There were no conflicts of interest between the authors and any other entity, either public, non-profit or private, with respect to this research.




