CVD and type 2 diabetes mellitus (T2DM) are typically characterised by oxidative stress, endothelial dysfunction and sub-clinical chronic inflammation( Reference Szmitko, Wang and Weisel 1 ). C-reactive protein (CRP) is an acute-phase reactant protein released by hepatocytes following stimulation by inflammatory cytokines, including IL-6( Reference Pepys and Hirschfield 2 ). Circulating markers of inflammation, such as CRP, TNF-α, IL-6 and IL-1 are associated with a high risk of CVD( Reference Mazidi, Heidari-Bakavoli and Khayyatzadeh 3 ). It has been suggested that knowledge of CRP levels could improve the prediction of CVD and T2DM occurrence( Reference Mazidi, Karimi and Rezaie 4 ). There is also growing evidence that the influence of diet on CVD occurs through mechanisms that include subclinical inflammation( Reference Mazidi, Heidari-Bakavoli and Khayyatzadeh 3 ). CRP might directly promote endothelial dysfunction by decreasing endothelial nitric oxide synthase expression and mRNA stability, stimulating endothelial lectin-like oxidised LDL receptor 1 expression, promoting reactive oxygen species production and enhancing endothelial apoptosis( Reference Mineo, Gormley and Yuhanna 5 ).
Fibrinogen (FG) is involved in the process of blood coagulation, serving as a major component in thrombosis, and is regarded as one of the inflammatory markers( Reference Stefanadi, Tousoulis and Papageorgiou 6 ). Correlations between FG and CVD risk have been widely investigated. Existing studies support the independent association of elevated FG level with atherosclerotic CVD( Reference Danesh, Lewington and Thompson 7 ), as well as recurrent CVD and mortality in patients with existing CVD( Reference Espinola-Klein, Rupprecht and Bickel 8 , Reference Becker, Cannon and Bovill 9 ).
A higher intake of fruits and vegetables is associated with a lower risk of CVD( Reference Hermsdorff, Zulet and Puchau 10 ), possibly due to the antioxidant properties of several phytochemicals and vitamins that are abundant in fruits and vegetables( Reference Pellegrini, Salvatore and Valtuena 11 ). Antioxidants, including α-tocopherol, retinol, β-carotene, vitamins D and E have been reported to affect oxidative stress or inflammatory markers in vitro ( Reference Kaur, Rao and Agrawal 12 ), in rodent models( Reference El-Mowafy, El-Mesery and Salem 13 ) and in epidemiological studies( Reference Suzuki, Inoue and Hashimoto 14 ). However, interventional studies in humans assessing the effects of a single antioxidant on CVD risk factors have been controversial( Reference Vivekananthan, Penn and Sapp 15 – Reference Katsiki and Manes 17 ).
In the present study, we aimed to evaluate the associations between serum antioxidant levels and inflammatory markers, including FG and CRP, in US adults aged ≥18 years, who took part in the National Health and Nutrition Examination Surveys (NHANES) between 2001 and 2002. Furthermore, we evaluated the impact of adiposity (assessed by BMI) on the link between serum antioxidant levels and inflammatory markers.
Methods
Population
The NHANES protocol has been extensively described( Reference Mazidi, Katsiki and Mikhailidis 18 ). This is an ongoing programme of cross-sectional surveys conducted periodically by the US National Center for Health Statistics (NCHS). Participants in NHANES (about 5000/year) are selected using a multistage probability sampling approach, with where relevant, oversampling of certain segments of the population. Surveys are approved by the NCHS Research Ethics Review Board and all participants provide informed consent. During these surveys, data on demographics, dietary and behavioural patterns are collected using questionnaires administered during home visits. The interview consists of questions on socio-demographic characteristics (age, sex, education, race and health insurance) and history of diagnosed medical conditions. Anthropometric measurements, physical examination and sample collection for biomarkers assays are performed by trained survey workers using mobile examination units. Height and weight, measured with participants in underwear, are used to calculate BMI as weight in kg divided by the square of height in m. Based on self-reported smoking status, participants are classified as current smokers or not( Reference Mazidi, Shivappa and Wirth 19 ). The NHANES data are reported per 2-year cycles and are made publicly available for any relevant purpose.
Biochemical assays
A blood sample was drawn from an antecubital vein. Serum concentrations of vitamins A (retinol) and E (α-tocopherol), two retinyl esters, and six carotenoids (α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin, combined lutein/zeaxanthin and trans-lycopene) were measured using HPLC with photodiode array detection( 20 ). Total serum 25-hydroxy (25(OH)) vitamin D was assayed using a RIA kit (DiaSorin)( Reference Mazidi, Michos and Banach 21 ). The CV was 7 %( Reference Mazidi, Michos and Banach 21 ). Glycated Hb was measured using a Tosoh A1C 2·2 Plus Glycohemoglobin Analyzer (Tosoh Bioscience). Fasting blood glucose (FBG) was measured by using a hexokinase enzymatic method. Insulin was measured using an ELISA immunoassay (Mercodia)( Reference Mazidi, Penson and Banach 22 ). Levels of total cholesterol (TC) and TAG were measured enzymatically; LDL-cholesterol was calculated according to the Friedewald equation( Reference Friedewald, Levy and Fredrickson 23 ). Serum CRP concentrations were measured by latex-enhanced nephelometry and serum uric acid (SUA) by the uricase–peroxidase technique( Reference Mazidi, Shivappa and Wirth 19 ). Based on the NHANES Laboratory Procedures Manual, total bilirubin concentration (mg/dl) in serum or plasma was measured using a timed-endpoint Diazo method, a colorimetric analysis at 520 nm, and the sensitivity was 0·1 mg/dl (1·71 μmol/l). Other laboratory-test details are available in the NHANES Laboratory/Medical Technologists Procedures Manual( Reference Mazidi, Shivappa and Wirth 19 ). CRP levels >3 mg/l was considered as an indicator of high CVD risk( Reference Pearson, Mensah and Alexander 24 ).
Statistical analysis
Data were analysed using SPSS complex sample module version 22.0 (IBM Corp.). We followed the Centers for Disease Control and Prevention guidelines for analysis of the complex NHANES data, accounting for the masked variance and using the proposed weighting methodology( Reference Mazidi, Shivappa and Wirth 25 , Reference Mazidi, Kengne and Mikhailidis 26 ). We used mean and standard error of mean for continuous measures and percentages for categorical variables. Adjusted (for age, sex, race, education, marital status, BMI, serum bilirubin, SUA, TAG, TC, FBG and smoking) logistic regressions were used to investigate the associations between antioxidants, CRP and FG, as well as the likelihood of ‘CVD risk’ with quarters of serum antioxidants (with the first quarter (Q1) considered as reference). Multi-collinearity for the multiple linear regressions was assessed with variance inflation factors (VIF) at each step( Reference Slinker and Glantz 27 ). Multi-collinearity was considered high for VIF >10( Reference Slinker and Glantz 27 ). Groups were compared using ANCOVA and χ 2 tests.
The SPSS macro for moderation model by Preacher and Hayes( Reference Preacher and Hayes 28 ) was used to investigate the effects of adiposity on the associations of antioxidants with CRP and FG. The application of this macro allowed to simultaneously test the moderation impact of adiposity, while adjusting for relevant extraneous factors. The approach also allowed the visualisation of the impact of each standard deviation change in the potential moderator on the relationship between independent and dependent variables. We tested for the presence of an effect of the adiposity adjusted model (age, sex, race, education, marital status, BMI, serum bilirubin, SUA, TAG, TC, FBG and smoking). All tests were two sided and P<0·05 was used to characterise statistically significant results.
Results
Overall, 784 participants were eligible for this analysis, including 372 (47·5 %) men. The mean age was 46·9 years overall, with no difference between men and women (47·2 v. 46·6 years, respectively; P=0·071). Demographic characteristics of the participants across quartiles of CRP and FG are shown in Table 1. Age increased from 40·9 (lowest quartile) to 48·1 (top quartile) years across increasing quartiles of CRP and from 41·5 to 49·1 years across increasing quarters of FG (P<0·0001 for all comparisons). For both CRP and FG, the proportion of women was higher in the top than in the lowest quartiles (P<0·001 for all comparisons). Significant differences were observed in the distribution of race, marital status and education, across quartiles of CRP and FG (Table 1).
Table 1 Demographic characteristics of the participants across quartiles (Q) of C-reactive protein (CRP) and fibrinogen levels (Mean values with their standard errors and percentages)
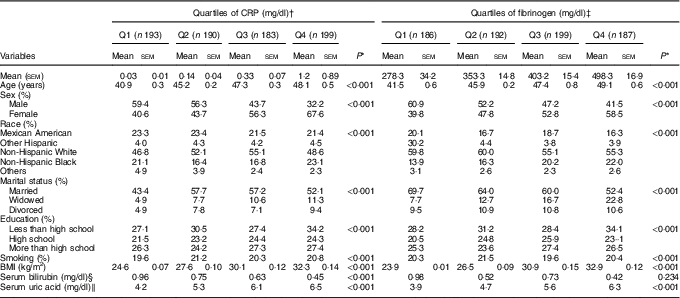
* Variables were compared across quartiles of CRP and fibrinogen using ANOVA or χ 2.
† To convert CRP in mg/dl to mg/l, multiply by 10.
‡ To convert fibrinogen in mg/dl to μmol/l, multiply by 0·0294.
§ To convert bilirubin in mg/dl to μmol/l, multiply by 17·1.
‖ To convert uric acid in mg/dl to μmol/l, multiply by 59·48.
Age, sex, race, education, marital status, BMI, serum bilirubin, SUA, TAG, TC, FBG and smoking-adjusted mean serum levels of antioxidants across quartiles of CRP and FG are shown in Table 2. Levels of α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin, combined lutein/zeaxanthin and retinol decreased across increasing quartiles of CRP and FG (P<0·001 for all comparisons), whereas concentrations of trans-lycopene and retinyl palmitate were reduced only across quartiles of CRP (P<0·001 for all comparisons). Levels of α-tocopherol and 25(OH) vitamin D significantly decreased only across increasing quartiles of FG (P<0·001 for all comparisons).
Table 2 Adjusted (for age, sex, race, education, marital status, BMI, serum bilirubin, serum uric acid, total cholesterol, TAG, fasting blood glucose and smoking) mean of serum antioxidants across quartiles (Q) of C-reactive protein (CRP) and fibrinogen levels (Mean values with their standard errors and percentages)
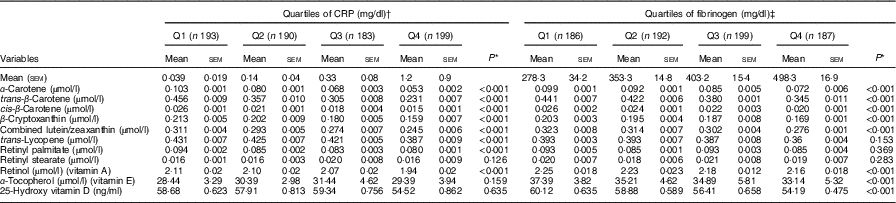
* P values for linear trend across quartiles. Variables were compared across quartiles of CRP and fibrinogen using ANCOVA.
† To convert CRP in mg/dl to mg/l, multiply by 10.
‡ To convert fibrinogen in mg/dl to μmol/l, multiply by 0·0294.
In multivariable linear regression models adjusted for age, sex, race, education, marital status, BMI, serum bilirubin, SUA, TAG, TC, FBG and smoking, a significant negative association was observed for α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin, combined lutein/zeaxanthin, trans-lycopene, retinyl palmitate, α-tocopherol, retinol and 25(OH) vitamin D with CRP (P<0·001 for all comparisons, Table 3). Furthermore, α-carotene, trans-β-carotene, cis-β-carotene, combined lutein/zeaxanthin, trans-lycopene, α-tocopherol, retinol and 25(OH) vitamin D were negatively associated with FG levels (P<0·001 for all comparisons). For example, a higher α-carotene level by 1 µmol/l correlated with 0·064 mg/dl lower CRP and 0·043 mg/dl lower FG levels (P<0·001 for all comparisons). Corresponding values were 0·084 and 0·039 mg/dl for each µmol/l higher trans-β-carotene level, 0·073 and 0·049 mg/dl for each µmol/l higher cis-β-carotene, 0·070 and 0·022 mg/dl for each µmol/l higher combined lutein/zeaxanthin, and 0·067 and 0·048 mg/dl for each µmol/l higher trans-lycopene (Table 3).
Table 3 Adjusted (for age, sex, race, education, marital status, BMI, serum bilirubin, serum uric acid, TAG, total cholesterol, fasting blood glucose and smoking) linear regression for the association between C-reactive protein (CRP) and fibrinogen levels with serum antioxidant vitamins (β-Coefficients and 95 % confidence intervals)
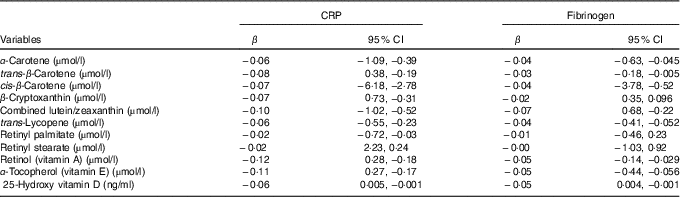
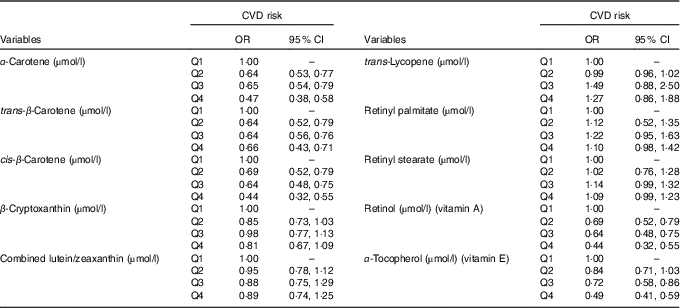
Table 4 shows the adjusted logistic regression analysis to determine CVD risk across quartiles of antioxidant vitamins levels. For α-carotene, trans-β-carotene, cis-β-carotene, vitamins A and E levels, the CVD risk decreased with increasing levels of these antioxidants. For example, participants in the top quartiles of vitamins A and E had 56 % (95 % CI 0·32, 0·55) and 51 % (95 % CI 0·41, 0·59) lower odds of CVD compared with participants in the first quartiles (Table 4).
Table 4 Multivariable logistic regression (adjusted for age, sex, race, education, marital status, BMI, serum bilirubin, serum uric acid, TAG, total cholesterol, fasting blood glucose and smoking) for the risk of CVD across quartiles (Q) of antioxidant vitamin levels (Odds ratios and 95 % confidence intervals)
In adjusted logistic regression analysis, BMI was a significant moderator of the link between CRP and α-carotene, trans-β-carotene, cis-β-carotene, vitamins A and E. For example, when levels of vitamin A (measured in µmol/l) changed from low (1·48) to high (1·98), the CRP in the low BMI category (mean −1sd, 22·4 kg/m2) changed from 0·31 to 0·96 (an increase of 0·65). In contrast, in the high BMI category (mean 1sd, 36·1 kg/m2), vitamin A (measured in µmol/l) changed from 0·34 to 1·12 (an increase of 0·78), suggesting that obesity may modulate the impact of vitamin A on CRP.
Discussion
In the present study, a significant inverse association was observed between serum several antioxidant vitamins and inflammatory markers (i.e. CRP and FG). Furthermore, individuals with a higher level of serum antioxidants had a lower risk of CVD (defined by CRP level). These observations were unaffected by the adjustment for several confounding factors, suggesting a potential protective effect of antioxidants against pathological processes involving subclinical inflammation. Furthermore, the link between CRP and antioxidant vitamins levels was mediated by BMI, suggesting a determining role of obesity in the occurrence of subclinical inflammation.
Consumption of selected fruits, vegetables, herbs and spices rich in antioxidants improved markers of oxidative stress and inflammation in a previous review( Reference Serafini and Peluso 29 ). However, there are data not supporting the use of these vitamins to reduce CVD risk( Reference Dagenais, Marchioli and Yusuf 30 ). In the Women’s Health Study( Reference Lee, Cook and Gaziano 31 ), no overall benefit was found for vitamin E in relation to major CVD events and total mortality. The Heart Outcomes Prevention Evaluation trial in people aged ≥55 years with CVD risk factors, showed no overall effect of antioxidant vitamins on CVD outcomes( Reference Sleight 32 ). Daily multivitamin consumption did not decrease major CVD events during a decade of follow-up of US men in the Physicians’ Health Study II( Reference Sesso, Christen and Bubes 33 ). A meta-analysis of randomised trials also reported no effect of antioxidant vitamin supplementation on major fatal and non-fatal CVD, as well as all-cause mortality( Reference Ye, Li and Yuan 34 ). Furthermore, vitamin E supplementation (at doses ≤400 IU/d) had no significant impact on inflammatory markers in postmenopausal women( Reference Carr, Khan and Adams-Huet 35 ). In contrast, ex vivo studies reported that vitamin E at doses of 600–1200 IU/d can significantly decrease the levels of inflammatory factors( Reference Devaraj, Li and Jialal 36 , Reference van Tits, Demacker and de Graaf 37 ).
In all, two studies examined the effects of the combination of vitamins C and E on CRP; none found a significant effect on CRP levels( Reference Block, Jensen and Dietrich 38 , Reference Bruunsgaard, Poulsen and Pedersen 39 ). However, they used different doses, that is, 182 mg α-tocopherol and 500 mg vitamin C( Reference Bruunsgaard, Poulsen and Pedersen 39 ), 371 mg α-tocopherol and 515 mg vitamin C( Reference Block, Jensen and Dietrich 38 ). Hartel et al. ( Reference Hartel, Strunk and Bucsky 40 ) found that vitamin C inhibits the lipopolysaccharide-induced IL-6 and TNF-α production, as well as IL-2 production after phorbol 12-myristate 13-acetate/ionomycin stimulation. It was suggested that vitamin C could decrease the level of oxidative stress and consequently inflammation, as oxidative damage leads to an inappropriate activation of the transcription NF-κB and subsequently to an overexpression of inflammatory proteins( Reference Baeuerle and Henkel 41 ). Similarly, vitamin C was shown to inhibit NF-κB activation( Reference Bowie and O’Neill 42 – Reference Perez-Cruz, Carcamo and Golde 44 ).
β-Carotene is the most investigated carotenoid for its antioxidant activity( Reference Ribeiro Nogueira, Ramalho and Lameu 45 , Reference Britton 46 ). A study which included 14 470 current smokers, ex-smokers and never smokers aged ≥18 years who participated in the third NHANES, evaluated the relationship between serum β-carotene and CRP and reported a strong and inverse association of serum β-carotene levels with CRP levels( Reference Erlinger, Guallar and Miller 47 ). Another study that used data from the MacArthur studies of successful aging (n 672), found a negative link between β-carotene and CRP concentrations( Reference Hu, Reuben and Crimmins 48 ). Recently, a study on eighty individuals (mean age=66·9 years) reported that the dietary intake of β-carotene does not significantly affect plasma or salivary CRP levels( Reference Gawron-Skarbek, Guligowska and Prymont-Przyminska 49 ). Another study examined cross-sectional correlations between CRP and plasma levels of α-tocopherol and β-carotene, reporting no association between plasma levels of α-tocopherol and CRP( Reference Il’yasova, Ivanova and Morrow 50 ). In contrast, plasma β-carotene was inversely related to CRP( Reference Il’yasova, Ivanova and Morrow 50 ).
Vitamin A plays a role in both pro-inflammatory cytokines such as IL-6 and upregulating IL-4 production (which is an anti-inflammatory marker)( Reference McDonald, Savy and Fulford 51 , Reference Kim 52 ). In this context, vitamin A supplementation (25 000 IU/d) was shown to reduce CRP levels in obese women( Reference Farhangi, Keshavarz and Eshraghian 53 ). In contrast, Filteau et al. ( Reference Filteau, Morris and Raynes 54 ) reported increased serum CRP concentrations after supplementation of 200 000 IU/d retinyl palmitate for 4 months in children with marginal vitamin A deficiency.
A study involving non-smoking participants from the third NHANES (n 4557 aged 25–55 years) reported that β-cryptoxanthin and FG were inversely associated( Reference Kritchevsky, Bush and Pahor 55 ). β-Cryptoxanthin is plentiful in foods that also tend to be high in vitamin C, which itself has been shown to be inversely related to FG levels( Reference Khaw and Woodhouse 56 ). Furthermore, Iribarren et al. ( Reference Iribarren, Folsom and Jacobs 57 ) found that sialic acid, which is elevated during the acute phase response, and white blood cell count, but not FG levels, were inversely associated with serum β-carotene levels. In multiple regression analysis, including a number of correlates of β-carotene levels, sialic acid remained inversely associated with β-carotene levels( Reference Iribarren, Folsom and Jacobs 57 ).
Due to the sampling strategy of NHANES, our findings can be generalised to the US population. However, a principal limitation of this analysis is its cross-sectional nature which cannot allow reliable definition of the direction of the effect of the observed associations. Although we accounted for several lifestyle factors, the possibility of the effect of unmeasured confounders remains. We included on CRP and FG, which may not capture all major inflammatory pathways. Although the sample size was acceptable, it included only a sub-sample of participants who took part in the targeted NHANES surveys. A major strength of this study is the use of objectively measured biomarkers in the analysis rather than relying on self-reported dietary intake.
In conclusion, the present study supports a possible beneficial effect of antioxidant vitamins on subclinical inflammation, mediated at least in part by the overall adiposity. To what extent, the observed associations may translate into a protective effect of antioxidant vitamins against pathological conditions involving subclinical inflammation, needs to be further investigated.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
M. M. contributed to the study concept and design, data analysis and interpretation, and drafting of the manuscript and contributed to the critical revision of the manuscript. A. P. K. contributed to the data analysis and interpretation, and drafting of the manuscript, contributed to the study concept and design. M. B., N. K. and D. P. M. contributed to the critical revision of the manuscript, contributed to the study concept and design. All the authors approved the final version of the paper.
N. K. has given talks, attended conferences and participated in trials sponsored by Amgen, Angelini, AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Novo Nordisk, Sanofi and WinMedica. D. P. M. has given talks and attended conferences sponsored by MSD, AstraZeneca and Libytec. The other authors have no conflict of interest to declare.







