Introduction
Idiopathic normal-pressure hydrocephalus (iNPH) is a clinical syndrome consisting of dilated cerebral ventricles along with the clinical triad of gait disturbance, cognitive disturbance (dementia), and/or urinary dysfunction. Cerebrospinal fluid (CSF) diversion through a surgically placed shunt is currently the standard method of treatment. Previously, the diagnosis of iNPH was made based on a positive response to a ventricular shunt, once ruling out possible causes of secondary NPH, such as subarachnoid hemorrhage or meningitis. However, in 2004, the Japanese Society of Normal Pressure Hydrocephalus published the first evidence-based guidelines on the diagnosis of iNPH,Reference Ishikawa 1 and it was recommended that persons with suspected iNPH be categorized as “definite,” “probable,” “possible,” and “unlikely.” The first widely accepted English-language evidence-based diagnostic criteria for idiopathic normal-pressure hydrocephalus were published in 2005Reference Relkin, Marmarou, Klinge, Bergsneider and Black 2 and followed the stratified approach of the Japanese guidelines. The Normal Pressure Hydrocephalus guidelines were updated in 2012.Reference Mori, Ishikawa and Kato 3
A shunt procedure is indicated for patients who fulfill the criteria of probable iNPH. Approximately 80% of patients diagnosed with probable iNPH will respond to a shunt procedure.Reference Toma, Papadopoulos, Stapleton, Kitchen and Watkins 4 , Reference Halperin, Halperin, Kurlan, Schwalb, Cusimano, Gronseth and Gloss 5 Due to the invasiveness and high adverse event rate (~11%Reference Halperin, Halperin, Kurlan, Schwalb, Cusimano, Gronseth and Gloss 5 ) of the shunt procedure, a tool to help predict which patients with probable iNPH will respond or not to a shunt procedure would be of clinical value. In recent studies with relatively large numbers of iNPH patients, it was found that no current radiological marker has been shown to predict shunt response with high sensitivity or specificity.Reference Virhammar, Laurell, Cesarini and Larsson 6 , Reference Kojoukhova, Koivisto and Korhonen 7 The 2012 clinical guidelines for iNPHReference Mori, Ishikawa and Kato 3 have recognized that there is potential for the use of CSF biochemical tests in the diagnosis and treatment of iNPH; however, the current evidence is not strong enough to recommend a change in clinical practice. With CSF sampling being routine in the diagnosis of iNPH, CSF biomarker analysis would be an extremely low-risk additional investigation.
The present review summarizes the current state of research into using preoperative CSF biomarkers as predictors of shunt response in iNPH patients.
Methods/Inclusion Criteria
To find all relevant articles, a comprehensive text-word search of Medline, Embase, and PsycINFO was conducted. All articles published as of 13 March 2017 were included.
In a preliminary literature search, it was found that shunt responsiveness was rarely a major focus of the currently available research studies and was therefore rarely mentioned in the titles or abstracts of research articles. Because of this, shunt responsiveness was not included in the literature search strategy. The full search strategy is outlined in Appendix 1 (see the Supplementary Materials).
A total of 344 unique citations were identified. All citations were reviewed by two independent reviewers. All discordant conclusions were resolved by a third independent reviewer. Abstract reviews were completed for all citations. Abstracts that made mention of NPH and CSF biomarkers were selected for full-article reviews. Full-article reviews were completed to determine which articles satisfied the following inclusion criteria:
-
1. must contain an iNPH study group as diagnosed using evidence-based guidelines
-
2. lumbar CSF biomarker analysis completed
-
3. shunt-responsive (SR) patients must be reported separately from shunt-nonresponsive (SNR) patients
-
4. iNPH and sNPH results must not be combined in the analysis
-
5. must consist of 10 or more iNPH patients
To be included in the review, a study had to specifically mention the inclusion of patients with idiopathic normal-pressure hydrocephalus, as diagnosed using evidence-based guidelines.Reference Ishikawa 1 - Reference Mori, Ishikawa and Kato 3 It has been shown that secondary NPH (sNPH), due to such causes as subarachnoid hemorrhage, meningitis, and traumatic brain injury, has a different CSF protein profile than iNPH.Reference Lee, Park and Back 8 , Reference Tullberg, Blennow, Månsson, Fredman, Tisell and Wikkelsö 9 Therefore, studies that combined both idiopathic and secondary NPH when reporting results were excluded. Studies that analyzed ventricular CSF were excluded, as sampling from this area is not considered a routine investigation in the diagnosis of iNPH, and ventricular CSF has been shown to have a different protein profile than lumbar CSF.Reference Pyykkö, Lumela and Rummukainen 10 , Reference Djukic, Spreer, Lange, Bunkowski, Wiltfang and Nau 11 The studies did not have to have a comparative group, but they had to report patient biomarker concentrations, separated into shunt-responsive and shunt-nonresponsive groups. Studies included in this review employed differing definitions of shunt responsiveness, including: any objective or subjective improvement of the iNPH symptom triad, improvement on the Modified Rankin Scale, and improvement in cognition as measured through a Mini-Mental Status Exam. In analysis of the evidence, we highlight these differing definitions and attempt to reconcile them when possible. See Figure 1 for a depiction of the study selection process.
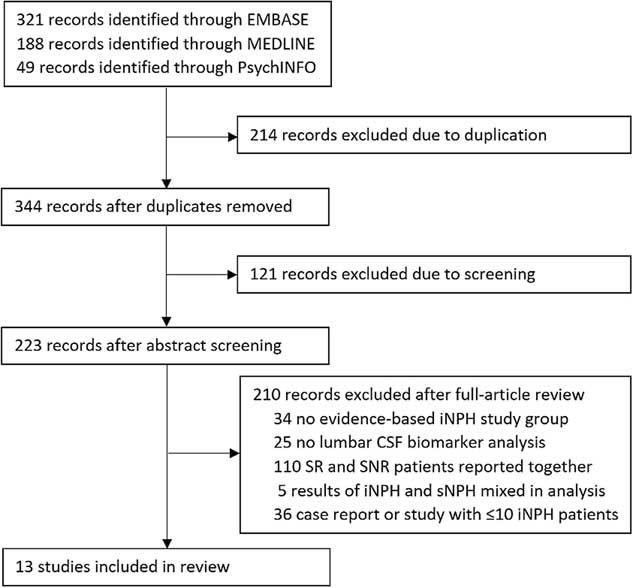
Figure 1 Study selection.
Analysis of Evidence
A total of 13 articles were found to satisfy the inclusion criteria. The quality of evidence in the included articles was ranked according to the American Academy of Neurology Clinical Practice Guidelines. 12 There were 6 class IV articles,Reference Nakajima, Miyajima and Ogino 13 - Reference Sosvorová, Vcelak, Mohapl, Vitku, Bicikova and Hampl 18 5 class III articles,Reference Pyykkö, Lumela and Rummukainen 10 , Reference Agren-Wilsson, Lekman and Sjöberg 19 - Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 and 2 class II articlesReference Nakajima, Miyajima and Ogino 23 , Reference Luikku, Hall and Nerg 24 relevant to the prognostic question.
The 13 relevant studies reported data on a total of 508 patients. Of the studies in which relevant patient data were available, the shunt response rate was 78.74%, and the mean age was approximately 74 years. These data led us to conclude that the patients included in our review are representative of the generally treated iNPH population.Reference Williams and Malm 25 In total, 37 unique CSF biomarkers were analyzed within the studies. Of the 37 CSF biomarkers, 23 were only analyzed within single studies (see Table 1). These biomarkers will be discussed briefly below, and the remaining 14 biomarkers, analyzed in multiple studies, will be discussed in greater detail further along in this article.
Table 1 CSF biomarkers analyzed in single studies

Interleukins (ILs)
Interleukins are a group of cytokines that play a role in regulating immune response and inflammatory reactions. Both antiinflammatory (IL-4 and IL-10), and proinflammatory (IL-1β, IL-6, IL-8, IL-17A, IL-21, IL-22, IL-31, IL-33) interleukins were investigated in the articles included in our review. Only IL-8 was directly compared between SR and SNR patients, and the levels were found to be not significantly different.Reference Pyykkö, Lumela and Rummukainen 10 SosvorováReference Sosvorová, Vcelak, Mohapl, Vitku, Bicikova and Hampl 18 found that IL-1β, IL-6, and, interestingly, IL-10 were significantly elevated in iNPH patients versus normal controls. That study did not account for comorbid conditions that could cause an elevation of interleukins. However, the levels of these interleukins decreased significantly in SR patients during lumbar drainage procedures. Because of this, SosvorováReference Sosvorová, Vcelak, Mohapl, Vitku, Bicikova and Hampl 18 proposed that IL-1β and IL-6 were elevated due to an inflammatory process occurring in the pathogenesis of iNPH and that IL-10 was possibly elevated as a normal body defense mechanism. However, all patients included in the study were classified as shunt responders, so their predictive value in differentiating SR patients from SNR patients cannot be determined. Further studies need to be done to determine the predictive value of interleukins in the workup of iNPH.
Other Proinflammatory Cytokines
Neuroinflammation and abnormal levels of CSF proinflammatory cytokines have been noted in various central nervous system diseases.Reference Chakraborty, Kaushik, Gupta and Basu 26 Monocyte chemoattractant protein-1 (MCP-1) and soluble CD40 ligand (sCD40L) were each investigated in one article included in our review. MCP-1 levels were directly compared between SR and SNR patients, and the levels were not found to be significantly different.Reference Pyykkö, Lumela and Rummukainen 10 sCD40L was included in an article in which all iNPH patients were classified as shunt responders, so the predictive value of sCD40L cannot be determined.Reference Sosvorová, Vcelak, Mohapl, Vitku, Bicikova and Hampl 18 However, no significant differences in sCD40L levels were found between iNPH patients and normal controls.
Markers of Neuronal Damage
As iNPH is a potentially reversible neurodegenerative disease, the presence of neuronal damage and the CSF biomarkers of such have been investigated in several studies.Reference Tullberg, Blennow, Månsson, Fredman, Tisell and Wikkelsö 9 , Reference Pyykkö, Lumela and Rummukainen 10 , Reference Agren-Wilsson, Lekman and Sjöberg 19 , Reference Tullberg, Blennow, Månsson, Fredman, Tisell and Wikkelsö 27 Sulfatide and myelin basic protein (MBP) were each investigated in one article included in our review. Sulfatide is a lipid component of the myelin sheath and has been used as a marker of white matter degradation.Reference Fredman, Wallin, Blennow, Davidsson, Gottfries and Svennerholm 28 MBP is a structural protein involved in the myelination of neurons in the central nervous system, and an increased CSF level is a well-established marker for myelination damage.Reference Whitaker 29 No statistically significant differences in sulfatide or MBP levels were found between SR and SNR patients.Reference Pyykkö, Lumela and Rummukainen 10 , Reference Agren-Wilsson, Lekman and Sjöberg 19
Neuroprotective Proteins
Neuroprotective proteins were investigated in three articles included in our review. Cystatin C, lipocalin-type prostaglandin D2 synthase (L-PGDS), and transthyretin are proteins involved in amyloid beta (Aβ) metabolism and transport, and they protect neuronal cells from Aβ toxicity and aggregation.Reference Kanekiyo, Ban and Aritake 30 - Reference Tizon, Ribe, Mi, Troy and Levy 32 In single studies, levels of L-PGDS, cystatin C, and transthyretin were found to be not significantly different between SR and SNR patients.Reference Nakajima, Miyajima and Ogino 13 , Reference Laiterä, Kurki and Pursiheimo 21 The physiological roles of transforming growth factor (TGF) and TGF beta receptor 2 (TBR-II) in the central nervous system are poorly understood, but they do exhibit neuroprotective functions.Reference Dobolyi, Vincze, Pál and Lovas 33 TGF-β1, TGF-β2, and TBR-II were investigated in one article in which all iNPH patients were classified as shunt responders, so the predictive value of these biomarkers cannot be determined.Reference Li, Miyajima, Jiang and Arai 15 Levels of TGF-β1 and TBR-II were significantly elevated in iNPH patients versus normal controls, and this warrants further investigation to determine their predictive value.
Others
Homocysteine, chitinase-3-like protein 1 (YKL-40), and Aβ43 were each investigated in single articles included in the present review. Homocysteine is an amino acid that has been shown to increase in a variety of neurological conditions, including multiple sclerosis, Parkinson’s disease, and dementia.Reference Ansari, Mahta, Mallack and Luo 34 Homocysteine was investigated in one article in which all iNPH patients were classified as shunt responders, so the predictive value of these biomarkers cannot be determined.Reference Sosvorová, Besták and Bicíková 17 Levels of homocysteine were significantly elevated in iNPH patients versus normal controls and warrant further investigation to determine the predictive value of homocysteine. YKL-40 is a glycoprotein whose function is not fully understood but is associated with inflammatory processes.Reference Rathcke and Vestergaard 35 YKL-40 levels were directly compared between SR and SNR patients, and their levels were not found to be significantly different.Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 Aβ43 is an amyloid beta peptide that is a key component of neuritic amyloid plaques. Aβ43 levels were directly compared between SR and SNR patients, and levels were not found to be significantly different.Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 Amyloid beta peptides are discussed further below.
The remaining 14 CSF biomarkers were analyzed in two or more studies and will be discussed further (see Table 2).
Table 2 CSF biomarkers analyzed in multiple studies

Aβ38, Aβ40, and Aβ42
Amyloid beta (Aβ) peptides are a key component of neuritic amyloid plaques, derived from amyloid precursor protein (APP), and are believed to trigger the pathogenesis of Alzheimer’s disease (AD). Low CSF levels of Aβ peptides, in particular Aβ42, are strongly associated with AD.Reference Olsson, Olsson and Lautner 36 Due to the age of the affected population, 25 to 40% of iNPH patients will have comorbid AD pathology.Reference Cabral, Beach and Vedders 37 - Reference Hamilton, Patel and Lee 39 In previous studies, less severe amyloid deposits correlated with better cognitive improvement after shunting in iNPH patients.Reference Hiraoka, Narita and Kikuchi 40 , Reference Koga 41 Due to their roles as core CSF biomarkers for neurodegeneration, Aβ peptides are among the most studied biomarkers for iNPH.
In this review, Aβ38 was analyzed in one class IVReference Nakajima, Miyajima and Ogino 13 and two class III articles,Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 , Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 Aβ40 was analyzed in two class III articles,Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 , Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 and Aβ42 was analyzed in two class IV,Reference Nakajima, Miyajima and Ogino 13 , Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 four class III,Reference Pyykkö, Lumela and Rummukainen 10 , Reference Agren-Wilsson, Lekman and Sjöberg 19 , Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 , Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 and one class II article.Reference Luikku, Hall and Nerg 24 In the studies included in our review, no statistically significant differences were found between SR and SNR patients for any of the Aβ peptides. A study by NakajimaReference Nakajima, Miyajima and Ogino 13 found that, with a cutoff value of >3.58, the Aβ38:Aβ42 ratio could predict postoperative cognitive improvement with a sensitivity of 77.8% and a specificity of 81%. The same study found that a cutoff of <14.6 for the Aβ42:p-tau ratio had a sensitivity of 76% and a specificity of 72.7%.Reference Nakajima, Miyajima and Ogino 13 However, that study only reported on postoperative cognitive improvement and did not report on improvements in gait or urinary function. Levels of Aβ peptides consistent with AD may have a role in predicting postoperative cognitive improvement, but the presence of these Aβ profiles does not definitively rule out iNPH patients from responding to a shunt procedure.
The available data do not support Aβ38, Aβ40, or Aβ42 individually as being reliable predictors of shunt responsiveness in iNPH patients. However, in combination with other CSF biomarkers, Aβ peptides (in particular, Aβ42), they may have prognostic value in the workup of iNPH.
sAPP, sAPPα, and sAPPβ
APP is a transmembrane protein that plays an integral role in a wide variety of neuronal functions.Reference Turner, O’Connor, Tate and Abraham 42 APP fragments, including APPα and APPβ, have neuroprotective and neurotrophic functions, and cleavage of APP also produces Aβ peptides.Reference Turner, O’Connor, Tate and Abraham 42 Levels of soluble APP (sAPP) and soluble APP fragments (sAPPα and sAPPβ) can be measured in CSF samples. In this review, sAPP was analyzed in two class IVReference Nakajima, Miyajima and Ogino 13 , Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 and one class III article,Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 sAPPα was analyzed in two class IVReference Nakajima, Miyajima and Ogino 13 , Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 and four class III articles,Reference Pyykkö, Lumela and Rummukainen 10 , Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 - Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 and sAPPβ was analyzed in two class IVReference Nakajima, Miyajima and Ogino 13 , Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 and four class III articles.Reference Pyykkö, Lumela and Rummukainen 10 , Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 - Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 Levels of sAPP were not found to be significantly different in shunt responders versus nonresponders in any of the included articles.
In five of the included studies, sAPPβ levels were not found to be significantly different in SR versus SNR patients.Reference Pyykkö, Lumela and Rummukainen 10 , Reference Nakajima, Miyajima and Ogino 13 , Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 - Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 However, one class III study found a very significant difference in sAPPβ levels.Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 In that study, reported sAPPβ levels in shunt-responsive patients were significantly lower than those reported in all other included studies.
In one class IIIReference Pyykkö, Lumela and Rummukainen 10 and one class IV article,Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 sAPPα levels were significantly different between SR and SNR patients and had moderately accurate predictive ability. Using a cutoff value of 198 ng/mL, MiyajimaReference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 found that sAPPα levels had a sensitivity of 66.7% and a specificity of 82.9% in predicting postoperative cognitive outcomes. In the remaining three class III articlesReference Moriya, Miyajima, Nakajima, Ogino and Arai 20 - Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 and one class IV article,Reference Nakajima, Miyajima and Ogino 13 sAPPα levels were not found to be significantly different. The available data do not support sAPP, sAPPα, or sAPPβ levels as being reliable predictors of shunt responsiveness in iNPH patients.
APL1β25, APL1β27, APL1β28
Amyloid precursor-like protein 1 β-derived (APL1β) peptides are similar to sAPP-derived peptides in sequence and function.Reference Coulson, Paliga, Beyreuther and Masters 43 , Reference Jacobsen and Iverfeldt 44 APL1β peptides do not deposit in the brain, and APL1β28 has been proposed as a surrogate biomarker for Aβ42.Reference Yanagida, Okochi and Tagami 45 In our review, APL1β25, APL1β27, and APL1β28 were each analyzed in two class III articles,Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 , Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 neither of which found any significant difference in levels between SR and SNR patients. The available data do not support APL1β25, APL1β27, or APL1β28 levels as being reliable predictors of shunt responsiveness in iNPH patients.
Tau and P-Tau-181
Tau is a structural protein that stabilizes microtubules and is abundant in the neurons of the central nervous system. CSF levels of tau protein and phosphorylated tau at threonine-181 (p-tau) are increased in patients with AD,Reference Olsson, Olsson and Lautner 36 and elevated CSF levels of tau are also found in such other neurodegenerative diseases as frontotemporal lobar degeneration and Creutzfeldt–Jakob disease.Reference van Harten, Kester and Visser 46 Due to their roles as core CSF biomarkers for neurodegeneration, tau and p-tau are among the most studied biomarkers for iNPH. In our review, tau was analyzed in one class IV,Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 three class III,Reference Pyykkö, Lumela and Rummukainen 10 , Reference Agren-Wilsson, Lekman and Sjöberg 19 , Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 and two class II articles,Reference Nakajima, Miyajima and Ogino 23 , Reference Luikku, Hall and Nerg 24 and p-tau was analyzed in two class IV,Reference Nakajima, Miyajima and Ogino 13 , Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 three class III,Reference Pyykkö, Lumela and Rummukainen 10 , Reference Agren-Wilsson, Lekman and Sjöberg 19 , Reference Moriya, Miyajima, Nakajima, Ogino and Arai 20 and one class II article.Reference Luikku, Hall and Nerg 24
Tau protein was not found to be significantly different in SR versus SNR patients in any of the included studies. However, in one class IV studyReference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 and one class II study,Reference Nakajima, Miyajima and Ogino 23 patients with high tau levels tended toward shunt nonresponsiveness.
Levels of p-tau were found to be significantly lower in two class IV studies, looking only at cognitive outcomes. NakajimaReference Nakajima, Miyajima and Ogino 13 found that a p-tau cutoff of ≤22 pg/mL was able to predict positive postoperative cognitive outcomes with a sensitivity of 77.8% and a specificity of 71.4%. That study did not report on improvements in gait or urinary function. In the remaining three class III articles, p-tau levels were not significantly different in SR versus SNR patients. Levels of tau and p-tau consistent with AD may play a role in predicting lack of postoperative cognitive improvement; however, the presence of these AD profiles does not definitively rule out iNPH patients from responding to a shunt procedure. Similar to the abovementioned Aβ peptides, the majority of studies investigating tau and p-tau did not account for comorbid conditions, such as AD, which could affect the CSF levels of these biomarkers. Due to the age of the affected population, 25-40% of iNPH patients will have comorbid AD pathologiesReference Cabral, Beach and Vedders 37 - Reference Hamilton, Patel and Lee 39 that cause abnormal levels of Aβ peptides, tau, and p-tau. Including these patients in analysis limits the ability of studies to determine whether abnormalities in these biomarkers are directly associated with the iNPH disease process. Though as discussed later on in this article, SR iNPH can exist concurrently with AD, and including these patients in analysis may yield results of greater real-world clinical relevance.
The available data do not support tau or p-tau, individually, as being reliable predictors of shunt responsiveness in iNPH patients. However, in combination with other CSF biomarkers, tau and p-tau may have prognostic value in the workup of iNPH.
NFL
Neurofilament light protein (NFL) is a major structural protein of axons, and it has been shown that NFL levels can be used as a marker of neuronal damage and neurodegeneration.Reference Rosengren, Karlsson, Karlsson, Persson and Wikkelsø 47 , Reference Gisslén, Price and Andreasson 48 Levels of NFL have previously been reported to correlate with shunt responsiveness.Reference Tullberg, Blennow, Månsson, Fredman, Tisell and Wikkelsö 9 In one study, NFL levels greater than 640 mg/L were identified as having a specificity of 100% but a low sensitivity of 17% for determining a positive outcome after shunt surgery.Reference Tullberg, Blennow, Månsson, Fredman, Tisell and Wikkelsö 9 That study, however, combined both secondary and idiopathic NPH in the analysis, and the diagnostic values found cannot be generalized to patients with probable iNPH.
NFL was analyzed in three class III articles included in our review.Reference Pyykkö, Lumela and Rummukainen 10 , Reference Agren-Wilsson, Lekman and Sjöberg 19 , Reference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 In a study by Pyykko,Reference Pyykkö, Lumela and Rummukainen 10 the levels of NFL were significantly higher in SNR versus SR patients. A study by Agren-WilssonReference Agren-Wilsson, Lekman and Sjöberg 19 found that, although NFL levels were not significantly different in SR versus SNR patients, SNR patients tended toward higher NFL levels. Higher NFL concentrations were found in iNPH patients with more severe symptoms, in line with its role as a marker of neuronal damage.Reference Agren-Wilsson, Lekman and Sjöberg 19 The remaining study by JeppssonReference Jeppsson, Höltta, Zetterberg, Blennow, Wikkelsø and Tullberg 22 found no significant differences in NFL levels. The available data suggest that NFL may have prognostic value in the evaluation of iNPH, though no study included in our review reported any predictive value.
LRG
Leucine-rich alpha-2-glycoprotein (LRG) is an inflammation-induced protein. It has been proposed as a biomarker for a variety of diseases, including ulcerative colitis, ovarian cancer, and pancreatic cancer.Reference Gisslén, Price and Andreasson 48 - Reference Andersen, Boylan and Jemmerson 50 However, studies investigating its use as a CSF biomarker for neurodegenerative diseases have been minimal.
LRG was analyzed in two class IV articlesReference Li, Miyajima, Mineki, Taka, Murayama and Arai 14 , Reference Li, Miyajima, Jiang and Arai 15 and one class II articleReference Nakajima, Miyajima and Ogino 23 included in our review. In one of the only class II studies included herein, NakajimaReference Nakajima, Miyajima and Ogino 23 found that elevated LRG levels correlate strongly with shunt responsiveness. Using receiver operating characteristic (ROC) analysis, his study found that, using an LRG cutoff of ≥67 ng/mL, SR and SNR patients were able to be differentiated with a sensitivity of 81.6% and a specificity of 78.6%. Using the same data, an LRG cutoff of ≥20 ng/mL was able to predict shunt response with a sensitivity of 100% and a specificity of 54.5% (i.e., 100% of responsive patients would be classified as SR, and 54.5% of SNR patients would be classified as SNR). The remaining class IV studies by LiReference Li, Miyajima, Mineki, Taka, Murayama and Arai 14 , Reference Li, Miyajima, Jiang and Arai 15 contained relatively few iNPH patients (15 and 21, respectively), and all were classified as SR. Because all iNPH patients were classified as SR, these studies do not directly add to determining the predictive value of LRG, though they do aid in evaluation of the remaining study by Nakajima.Reference Nakajima, Miyajima and Ogino 23 The studies investigating LRG did not account for comorbid conditions that could cause an elevated LRG. However, the studies by LiReference Li, Miyajima, Mineki, Taka, Murayama and Arai 14 , Reference Li, Miyajima, Jiang and Arai 15 found that levels of LRG were significantly elevated in iNPH patients compared to age-related controls, suggesting that the LRG elevation is due to the iNPH disease process.
The mean LRG levels in SR patients found in the studies by LiReference Li, Miyajima, Mineki, Taka, Murayama and Arai 14 , Reference Li, Miyajima, Jiang and Arai 15 are approximately two orders of magnitude lower than those found by NakajimaReference Nakajima, Miyajima and Ogino 23 at 1.14 and 1.05 ng/mL versus 96.8 ng/mL, respectively. This difference could be explained through the studies using different, nonstandardized enzyme-linked immunosorbent assays (ELISAs), or due to an analysis error. Due to this difference, no direct comparison can be made between these studies. The available data suggest that LRG levels may have prognostic value in the evaluation of iNPH, though additional high-quality research is required.
TNF-α
Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine that is central in a variety of disease states, including rheumatoid arthritis, psoriasis, inflammatory bowel disease, systemic infection, and ischemia-reperfusion injury.Reference Strieter, Kunkel and Bone 52 , Reference Esposito and Cuzzocrea 53 With abnormal levels of proinflammatory cytokines being present in various neurological diseases, TNF-α has been investigated in numerous studies of iNPH.Reference Lee, Park and Back 8 , Reference Pyykkö, Lumela and Rummukainen 10 , Reference Sosvorová, Vcelak, Mohapl, Vitku, Bicikova and Hampl 18 , Reference Leinonen, Menon and Carroll 54 TNF-α was analyzed in one class IVReference Sosvorová, Vcelak, Mohapl, Vitku, Bicikova and Hampl 18 and one class III articleReference Pyykkö, Lumela and Rummukainen 10 included in our review.
PyykkoReference Pyykkö, Lumela and Rummukainen 10 found no significant differences in TNF-α levels between SR and SNR patients. In a study by Sosvorová,Reference Sosvorová, Vcelak, Mohapl, Vitku, Bicikova and Hampl 18 all iNPH patients were classified as shunt responders. However, no significant differences in TNF-α levels were found between iNPH patients and normal controls. The available data do not support TNF-α levels as being reliable predictors of shunt responsiveness in iNPH patients.
Discussion
The purpose of this review was to summarize the current state of research into using preoperative CSF biomarkers to predict treatment response in iNPH patients. Through our review, we found that the available evidence is greatest for the use of Aβ42, tau, p-tau, NFL, and LRG in predicting shunt response in iNPH patients. There is minimal evidence for the use of TGF-β1, TBR-II, homocysteine, and interleukins (in particular IL-1β, IL-6, and IL-10). However, the available evidence suggests that these biomarkers warrant further investigation. We found that there was no available evidence for the use of Aβ38, Aβ40, Aβ43, APL1β25, APL1β27, APL1β28, sAPP, aAPPα, sAPPβ, TNF-α, MCP-1, sCD40L, sulfatide, MBP, L-PGDS, cystatin C, transthyretin, TGF-β2, and YKL-40. There are other CSF biomarkers that are commonly investigated in other neurological conditions that have not been investigated for iNPH. These include alpha-synuclein (α-synuclein), interferon gamma (IFN-γ), neuron-specific enolase (NSE), and visinin-like protein-1 (VILIP-1). Alpha-synuclein is a major constituent of Lewy bodies and has been investigated widely as a biomarker for Parkinson’s disease.Reference Schulz-Schaeffer 55 , Reference Malek, Swallow, Grosset, Anichtchik, Spillantini and Grosset 56 IFN-γ is a proinflammatory cytokine, and, as discussed above, abnormal levels of CSF proinflammatory cytokines have been noted in various central nervous system diseases.Reference Chakraborty, Kaushik, Gupta and Basu 26 NSE is a glycolytic enzyme found in neuronal and neuroendocrine tissues and has been investigated as a marker of neurodegeneration in Alzheimer’s disease.Reference Olsson, Olsson and Lautner 36 VILIP-1 is a neuronal calcium sensor protein and has also been investigated as a marker of neurodegeneration in Alzheimer’s disease.Reference Olsson, Olsson and Lautner 36
It is expected that no single CSF biomarker will be able to reliably predict shunt-responsive patients with high sensitivity and specificity. This is because the lack of treatment response in patients is likely caused by many different pathologies, including progressed and irreversible iNPH, or such other concurrent neurodegenerative diseases as Alzheimer’s disease, Parkinson’s disease, or dementia with Lewy bodies. It is also not yet known whether iNPH represents a distinct clinicopathological entity or rather a heterogeneous syndrome. A combination approach, such as that used by Nakajima,Reference Nakajima, Miyajima and Ogino 23 will likely increase the clinical value of biomarkers in predicting shunt responsiveness. Nakajima combined cutoff values for both LRG and tau to maximize sensitivity and specificity. Because of the potential for significant improvements in quality of life with shunt surgery for patients with SR iNPH, it is important that patients who would otherwise be good surgical candidates, not be refused shunt surgery based on false-negative biomarker analysis. For this reason, sensitivity of biomarker profiles should be prioritized over specificity in future studies (see Table 3).
Table 3 Summary of biomarker evidence

Through this review, it was found that iNPH patients with biomarker profiles consistent with AD tended toward lower postoperative cognitive improvement, which is not an unexpected result. Decreased CSF levels of Aβ42 and increased CSF levels of tau and p-tau are strongly associated with AD.Reference Olsson, Olsson and Lautner 36 The sensitivities and specificities of these biomarker profiles approached 80% in predicting postoperative cognitive improvement in iNPH patients. It is important to note, however, that iNPH patients can exhibit postoperative cognitive improvement, as well as gait and urinary symptom improvement, even in the face of biomarkers consistent with AD, confirming that iNPH is a syndrome that can exist with AD.
A limitation of our review article is that the majority of available data are presented such that predictive values cannot be calculated. Only two class IV studiesReference Nakajima, Miyajima and Ogino 13 , Reference Miyajima, Nakajima, Ogino, Miyata, Motoi and Arai 16 and one class II studyReference Nakajima, Miyajima and Ogino 23 included prognostic sensitivities and specificities. Studies that report whether biomarkers are statistically significant or not between SR and SNR patients do have great value in guiding future research. However, future studies should focus on determining and verifying the predictive values (specificity/sensitivity) of these CSF biomarkers, so that they might be of clinical value. The majority of the included studies had the primary purpose of determining biomarker value in the differential diagnosis of iNPH. Many of the biomarkers that showed no predictive value in determining shunt responsiveness were shown to be of value in the differential diagnosis of iNPH, though these data were not analyzed in this review. Another limitation of our review article is that the included studies used varying definitions to determine shunt responsiveness, making it difficult to directly compare the results of studies. In four of the studies included in our review, all iNPH patients were classified as shunt-responsive.Reference Li, Miyajima, Mineki, Taka, Murayama and Arai 14 , Reference Li, Miyajima, Jiang and Arai 15 , Reference Sosvorová, Besták and Bicíková 17 , Reference Sosvorová, Vcelak, Mohapl, Vitku, Bicikova and Hampl 18 These studies do not directly add to determining the predictive value of the biomarkers analyzed, though, as discussed in the analysis of LRG above, they can aid in the evaluation and validation of the remaining studies.
Current studies looking into iNPH biomarkers have been limited by their small numbers. Common to all studies evaluating prognostic outcomes of iNPH shunt patients is that the number of patients who are shunt-nonresponsive is often too low to draw any definite conclusions. This was particularly relevant for our review, as 8 of the 13 included studies had 10 or fewer shunt-nonresponsive patients. Multiple research networks, including the Adult Hydrocephalus Clinical Research Network in North America, are working on developing CSF biobanks for adult hydrocephalus patients. Future studies into CSF biomarkers should take advantage of such biobanks so as to achieve greater numbers, avoid single-center bias, and achieve greater reproducibility. This will be especially important when conducting research into the relationship between CSF biomarkers and shunt responsiveness where single-site/single-surgeon bias may occur.
Conclusions
Aβ42, tau, p-tau, NFL, and LRG have the greatest amount of evidence for their predictive value in determining shunt responsiveness in iNPH patients. There is minimal evidence for the use of TGF-β1, TBR-II, homocysteine, and interleukins (in particular IL-1β, IL-6, and IL-10). However, the available evidence suggests that these biomarkers warrant further investigation. Future research should be guided by, but not limited to, these biomarkers. Future studies should focus on determining and verifying the predictive value (specificity/sensitivity) of these CSF biomarkers so that they may be of clinical value. Importantly, SR iNPH may coexist with biomarker profiles consistent with AD.
Disclosures
Tyler Pfanner and Stephanie Borchert have nothing to disclose. Dr. Henri-Bhargava reports grants from Lilly and Astrazeneca via Paraxel, from Boehringer Ingelheim, from Roche, and from TauRx; and personal fees from Merck and the Tapestry Foundation; outside the submitted work.
Statement of Authorship
Study concept and design: TP, AHB. Literature review: TP, AHB, SB. Interpretation of data: TP, AHB. Drafting of manuscript and revisions: TP, AHB.
Supplementary material
To view the supplementary materials for this article, please visit https://doi.org/10.1017/cjn.2017.251






