The primary role of the human growth hormone insulin-like growth factor (IGF) axis is the regulation of both prenatal and postnatal growth( Reference Daughaday and Rotwein 1 , Reference Jones and Clemmons 2 ), and IGF binding proteins (IGFBP) are essential modulators of the biological actions of IGF( Reference Clemmons 3 ). Besides the regulation of normal growth and ageing, the IGF system is also involved in carcinogenesis( Reference Khandwala, McCutcheon and Flyvbjerg 4 ). Circulating concentrations of IGF system components are determined by both genetic factors (40–60 %, polymorphisms, imprinting) as well as dietary and lifestyle factors (e.g. diet, smoking, physical activity and others)( Reference Vrieling 5 ).
Carotenoids, such as α-carotene, β-carotene and β-cryptoxanthin, are known as provitamin A( Reference Suzuki, Ito and Hashimoto 6 ). Dietary β-carotene is present at relatively high concentrations in carrots and yellow and green leafy vegetables; α-carotene is present in carrots and red palm oil; and β-cryptoxanthin is found in sweet red pepper, oranges, tangerines and papaya( Reference Holden, Eldridge and Beecher 7 ). Six species of carotenoids comprise about 60–70 % of the total carotenoid pool in human plasma( Reference Barua, Kostic and Olson 8 , Reference Khachik, Beecher and Goli 9 ). The potential of carotenoids to modulate immune responses has been demonstrated in vivo and in cell models( Reference Chew and Park 10 ). Besides their antioxidant activity( Reference Bendich and Olson 11 ), investigators have proposed that carotenoids affect cell–cell communications( Reference Bertram 12 ) and have effects on membrane structure and signal transduction pathways( Reference Chew and Park 10 ). Large numbers of epidemiological studies have explored a possible association between carotenoid intake and reduced risk of CVD and cancer( Reference Van Poppel 13 – Reference Mayne 18 ), such as breast cancer( Reference Eliassen, Hendrickson and Brinton 19 ), nasopharyngeal carcinoma( Reference Polesel, Negri and Serraino 20 ) or urothelial cell carcinoma( Reference Ros, Bueno de Mesquita and Kampman 21 , Reference Miller and Snyder 22 ).
Lycopene, an acyclic non-provitamin A carotene, has received considerable attention for its possible role in cancer prevention, especially prostate cancer( Reference Khachik, Carvalho and Bernstein 23 – Reference Giovannucci 25 ). Lycopene is a tomato-derived substance, but is also present in watermelon, pink grapefruit, guava, papaya and apricots( Reference Clinton 26 ) and accounts for about 50 % of the carotenoids found in human blood; thus, it is the predominant carotenoid. It is a natural fat-soluble pigment and the most potent singlet oxygen quencher and free radical scavenger among all natural carotenoids( Reference Bhuvaneswari and Nagini 27 ). Therefore, lycopene is thought to decrease cancer risk through a reduction in oxidative damage( Reference Voskuil, Vrieling, van't Veer, Kampman and Rookus 28 ). In animal models, effects of lycopene on the endocrine IGF system, i.e. increased serum IGFBP-3 and decreased serum IGF-1:IGFBP-3 ratio, have been described( Reference Liu, Lian and Smith 29 ). Lycopene may inhibit activation of the IGF-1 receptor( Reference Karas, Amir and Fishman 30 ), alter IGF-1-stimulated cell proliferation in vitro ( Reference Karas, Amir and Fishman 30 , Reference Levy, Bosin and Feldman 31 ), induce cell cycle arrest( Reference Clinton 26 , Reference Voskuil, Vrieling, van't Veer, Kampman and Rookus 28 ), modulate intercellular communication via gap junction mechanisms( Reference Stahl, von Laar and Martin 32 , Reference Zhang, Cooney and Bertram 33 ), and have strong provitamin A activity via RAR-retinoid-X receptors (RXR) signalling pathways( Reference Aydemir, Carlsen and Blomhoff 34 – Reference Aydemir, Kasiri and Birta 36 ).
Based on the observations that both carotenoids and components of the IGF system have been found to be associated with cancer risk in some studies and potential associations between lycopene, as the most abundant carotenoid, and the IGF system in in vitro and in vivo studies, it was the aim of our study to examine the associations between circulating levels of carotenoids and fruit and vegetable consumption and serum concentrations of IGF-1, IGFBP-3 and their molar ratio in a cross-sectional US study.
Methods
Study population and data collection
The Third National Health and Nutrition Examination Survey (NHANES III) is a nationally representative sample of civilian non-institutionalised individuals conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention from 1988 to 1994( 37 ). This cross-sectional study was designed to collect the health and nutrition information of Americans aged 2 months and older through a structured household interview, serum collection and a physical examination in a mobile examination centre. In NHANES III, 39 695 persons were recruited( 37 ). The protocols for the conduct of NHANES III were approved by the Institutional Review Board of the National Center for Health Statistics, US Centers for Disease Control and Prevention. Informed consent was obtained from all participants.
In NHANES III, a FFQ over a 1-month reference period was to assess habitual diet, and we computed total fruit (four food items) and total vegetable (eleven food items) consumption based on the FFQ information. However, since only consumption frequency, but not portion sizes, has been assessed, we were not able to adjust for energy, macro- or micronutrient intake in our analysis.
Ascertainment of carotenoids, insulin-like growth factor-1 and insulin-like growth factor binding protein-3
Serum concentrations of α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin were determined using isocratic HPLC-based methods with detection at three different wavelengths (300, 325 and 450 nm; Waters HPLC System). Because these methods do not discriminate lutein from zeaxanthin, the combined concentration of lutein and zeaxanthin is used in analyses. Median interassay CV were 9·4 % for α-carotene, 7·0 % for β-carotene, 8·7 % for β-cryptoxanthin, 7·7 % for lycopene and 11·0 % for lutein/zeaxanthin( Reference Gunter, Lewis and Koncikowski 38 ). IGF-1 and IGFBP-3 concentrations were assessed in serum samples of NHANES III participants, 20+ years old (n 6061) from the NHANES III study population, who had participated in a morning examination( Reference Berrigan, Potischmann and Dodd 39 ). Individuals who participated in the morning session provided a blood sample after a mean overnight fast of approximately 11 h. Serum IGF-1 concentrations were available for 2742 men and 3316 women. Three persons were excluded with missing data. Serum samples were sent to the Diagnostic Systems Laboratories (DSL) in Webster, TX, which used the IGF-1 enzyme-linked immunosorbent assay (DSL 10-5600) and the IGFBP-3 immunoradiometric assay (DSL 6600) to measure serum concentrations of these biomarkers. Data for storage history and quality control for the 6061 serum samples from NHANES III have been reported in another study( Reference Berrigan, Potischmann and Dodd 39 ). The molar ratio of IGF-1:IGFBP-3 was computed as an indicator of free IGF-1 concentration( Reference Berrigan, Potischmann and Dodd 40 ).
Statistical analysis
For the characterisation of the study population, medians and interquartile ranges of all continuous parameters and percentages of all categorical parameters by sex were computed. Linear regression was used to examine the associations of fruit and vegetable consumption and serum concentrations of carotenoids (independent variables) with serum concentration of IGF-1, IGFBP-3 and their molar ratio (IGF-1:IGFBP-3) (dependent variables). Because serum carotenoid concentrations were not normally distributed in the study population, these values were transformed using the natural logarithm. Thus, we computed geometric mean concentrations of carotenoids by quintiles of IGF-1 and IGFBP-3, separately for men and women. We decided to stratify by sex a priori because IGF-1 and IGFBP-3 concentrations differ between men and women. For fruit and vegetable consumption, which were normally distributed, we computed arithmetic means by sex. In the linear regression models, we controlled for age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), smoking status (current, former or never smoker), alcohol consumption (non-consumer, up to once per week, less than daily but more than once/week, at least once per d), BMI (continuous) and serum cholesterol concentration (continuous). These variables were chosen a priori based on a literature review. Trend tests were performed by assigning to each individual the value 1 to 5 for the concentration/consumption category (1–5) into which the subject fell. We modelled this term as a continuous variable and the coefficient was evaluated by the Wald test. All tests were two-sided; P values <0·05 were considered to be statistically significant.
All statistical analyses were performed using SUDAAN( Reference Shah, Barnwell and Bieler 41 ) as implemented in SAS v. 9.1 (SAS Institute) software and weighted to take into account over-sampling, refusal, selection probabilities and differences from the general US population( 37 ). The protocols for the conduct of NHANES III were approved by the Institutional Review Board of the National Center for Health Statistics, US Centers for Disease Control and Prevention. Informed consent was obtained for all participants( Reference Giovannucci 25 ).
Results
Of the 2742 men included in this analysis, 77·6 % were non-Hispanic white, 9·5 % non-Hispanic black and 5·3 % Mexican-American; of the 3316 women, 76·5 % were non-Hispanic white, 11·3 % non-Hispanic black and 4·8 % Mexican-American. Table 1 shows baseline characteristics of study participants and the baseline median concentrations of carotenoids, IGF-1 and IGFBP-3. Men were younger and had higher BMI than women. Men were more likely to be current smokers and current drinkers of alcohol than women. Serum concentrations of all carotenoids but serum lycopene tended to be higher in women than in men. Serum IGF-1 concentrations tended to be higher and IGFBP-3 lower in men than in women.
Table 1. Baseline characteristics of study participants in the Third National Health and Nutrition Examination Survey (NHANES III) by sex (Percentages, medians and interquartile ranges or mean values and 95 % confidence intervals)
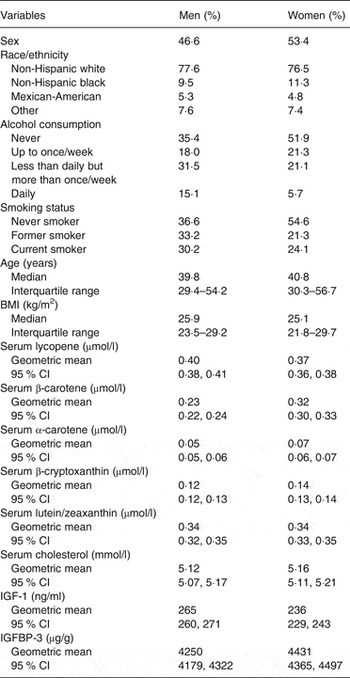
IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein.
In both sexes, serum IGF-1 and IGFBP-3 concentrations increased with increasing serum concentrations of all carotenoids (Table 2). Statistically significantly positive associations were observed for serum concentrations of lycopene, β-carotene, α-carotene, β-cryptoxanthin and lutein/zeaxanthin with IGF-1 in both men and women (all P trend <0·05). Overall, the increase in IGF-1 across quintiles of β-carotene, α-carotene and lutein/zeaxanthin was more pronounced among women than among men, whereas the increase in IGF-1 across quintiles of lycopene and β-cryptoxanthin was more pronounced among men.
Table 2. Comparison of serum insulin-like growth factor-1 (IGF-1; ng/ml), serum insulin-like growth factor binding protein-3 (IGFBP-3; μg/g) concentrations and their molar ratio according to categories of serum concentrations of carotenoids and total fruit and vegetable intake among men and women (Geometric means and 95 % confidence intervals)
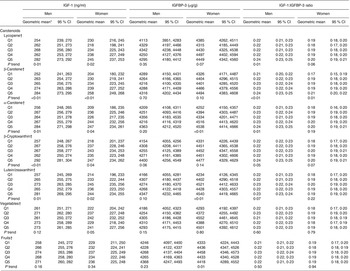
Q, quintile.
* Adjusted for age, BMI, race/ethnicity, smoking, alcohol intake and serum total cholesterol concentration.
† Cutpoints of quintiles are as follows: α-carotene 0·02, 0·06, 0·07, 0·11 µmol/l; β-carotene 0·13, 0·20, 0·32, 0·50 µmol/l; β-cryptoxanthin 0·09, 0·13, 0·18, 0·25 µmol/l; lutein/zeaxanthin 0·23, 0·32, 0·40, 0·53 µmol/l; lycopene 0·22, 0·32, 0·43, 0·56 µmol/l; fruit 6, 14, 30, 44 times per month; vegetables 31, 49, 71, 101 times per month.
Statistically significantly positive associations between serum concentrations of α-carotene and lutein/zeaxanthin with IGFBP-3 were observed in women (all P trend <0·05), but not in men. IGFBP-3 concentrations in men and women tended to increase with increasing lycopene, β-carotene and β-cryptoxanthin concentrations, but test for trend was not statistically significant (all P trend <0·05).
The IGF-1:IGFBP-3 molar ratio showed statistically significant positive associations with serum concentrations of lycopene, β-carotene and α-carotene in men, but no associations were observed for β-cryptoxanthin and lutein/zeaxanthin. In women, we only observed an association of the IGF-1:IGFBP-3 ratio with β-carotene concentration.
Only fruit intake was significantly positively associated with IGFBP-3 concentrations in women, and there was no significant association in men. Results for IGF-1 concentrations or the IGF-1:IGFBP-3 molar ratio were non-significant in both sexes. The analyses of the association of IGF-1, IGFBP-3 or the IGF-1:IGFBP-3 molar ratio with intake of vegetables did not show any statistically significant finding in women or men (all P trend >0·05).
Discussion
Our study was driven by the observation from animal and cell models that carotenoids, in particular lycopene, might beneficially influence components of the IGF system( Reference Liu, Lian and Smith 29 ). Prior to our analysis, twenty epidemiological studies had been published since 1999 that examined possible associations of carotenoid intake or circulating carotenoid concentrations, and consumption of fruits and vegetables with circulating concentrations of IGF-1 and IGFBP-3 (Supplementary Table S1). Of the twenty studies, eleven showed a link between one or more carotenoids, vegetables or fruits and the IGF system with either a positive or negative association. However, the results, as discussed below, were not consistent for carotenoid subgroups or nutrients and partly contradictory.
Associations with insulin-like growth factor-1
In the NHANES III analysis, higher serum concentrations of carotenoids were associated with higher IGF-1. This is in contrast to the theory that high carotenoid concentrations may protect from cancer by decreasing circulating levels of IGF-1. Our positive associations of serum lycopene concentrations with IGF-1 concentrations in men and women contrast with three other studies (case–control or intervention study), which found an inverse relationship between lycopene (supplement use or serum level) and IGF-1 concentrations( Reference Mucci, Tamimi and Lagiou 42 – Reference Walfisch, Walfisch and Kirilov 44 ) and thirteen studies that did not find any association (see Supplementary Table S1). All three studies with inverse associations had a very small number of participants ranging from twenty to 112 individuals and the two intervention studies( Reference Riso, Brusamolino and Martinetti 43 , Reference Walfisch, Walfisch and Kirilov 44 ) had very short treatment duration of 10–26 d. The positive association between serum lycopene concentrations and IGF-1 concentrations in our analysis might be due to differences in adjustment( Reference Mucci, Tamimi and Lagiou 42 , Reference Walfisch, Walfisch and Kirilov 44 ) or the selection of study participants( Reference Riso, Brusamolino and Martinetti 43 ).
The results of our analysis are in part consistent with those of Suzuki et al.( Reference Suzuki, Ito and Hashimoto 6 ), who observed significantly higher serum α-carotene, β-carotene and β-cryptoxanthin with increasing serum IGF-1 concentrations among women, but not men of a Japanese observational study. Our findings for associations with IGF-1 concentrations did not differ by sex. In contrast to our result and that of Suzuki et al.(6), one epidemiological study showed that serum β-carotene concentrations were inversely related to IGF-1 concentrations( Reference Norat, Dossus and Rinaldi 45 ). In addition, positive associations with serum IGF-1 concentrations were found for carotene( Reference Maruyama, Iso and Ito 46 ), α-carotene( Reference Suzuki, Ito and Hashimoto 6 , Reference Graydon, Young and Yarnell 47 ), β-carotene( Reference Suzuki, Ito and Hashimoto 6 ), β-cryptoxanthin( Reference Suzuki, Ito and Hashimoto 6 , Reference Holmes, Pollak and Willett 48 ) and for fruit intake( Reference Maruyama, Iso and Ito 46 ), but most other studies (n 12; Supplementary Table S1) showed no association with any carotenoid, fruit or vegetable intake and IGF-1.
Associations with insulin-like growth factor binding protein-3
Positive associations with IGFBP-3 concentrations were found for lycopene( Reference Holmes, Pollak and Willett 48 , Reference Vrieling, Voskuil and Bonfrer 49 ), vegetables( Reference McGreevy, Hoel and Lipsitz 50 ) and fruits( Reference Maruyama, Iso and Ito 46 ), which is in line with the observation that higher IGFBP-3 concentrations were related to decreased risk of several cancers( Reference Hankinson, Willett and Colditz 51 – Reference Yu, Spitz and Mistry 53 ). In contrast, an inverse relationship with serum IGFBP-3 concentrations was reported in two case–control studies for lycopene, lutein/zeaxanthin( Reference Suzuki, Ito and Hashimoto 6 ) and for vegetable intake( Reference Maruyama, Iso and Ito 46 ). The majority of studies (n 12), though, showed no association of any carotenoid examined with IGFBP-3 (Supplementary Table S1). The positive relationships of serum α-carotene and lutein/zeaxanthin concentrations with IGFBP-3 concentrations observed in our analysis in woman are new and in contrast to Suzuki et al.( Reference Suzuki, Ito and Hashimoto 6 ), who found an inverse relationship of serum lutein/zeaxanthin concentrations with IGFBP-3 in men. It should be kept in mind that α-carotene concentrations in serum are generally very low and rather variable( Reference Cantilena, Stukel and Greenberg 54 ); thus it cannot be excluded that the observed association is due to chance. Our results are in line with nine studies that reported no significant associations between lycopene and IGFBP-3 (Supplementary Table S1), although also inverse( Reference Suzuki, Ito and Hashimoto 6 ) and positive associations( Reference Holmes, Pollak and Willett 48 , Reference Vrieling, Voskuil and Bonfrer 49 ) have been reported.
Associations with the molar ratio (insulin-like growth factor-1:insulin-like growth factor binding protein-3)
In epidemiological studies, the IGF-1:IGFBP-3 molar ratio has been used as an approximate index of ‘free’, bioactive IGF-1, since IGFBP-3 is the main binding protein of IGF-1 in the circulation( Reference Berrigan, Potischmann and Dodd 40 , Reference Hankinson, Willett and Colditz 51 – Reference Yu, Spitz and Mistry 53 ). However, the biological effects of the different IGFBP on IGF-1 bioactivity are still relatively unknown. Similar to IGFBP-3, IGFBP-1 and -2 may also reduce bioactive IGF-1 by binding to it and making it unavailable for the IGF-1 receptor. On the other hand, IGFBP-1 and -2 allow the transport of IGF-1 out of the bloodstream, which may result in increased IGF-1 concentrations at the tissue level( Reference Vrieling, Voskuil and Bueno de Mesquita 55 ).
In our analysis, higher molar ratios of IGF-1:IGFBP-3 were related to higher serum concentrations of lycopene, β-carotene and α-carotene in men and to higher β-carotene concentration in women. These findings are in contrast to four other studies( Reference Mucci, Tamimi and Lagiou 42 – Reference Walfisch, Walfisch and Kirilov 44 , Reference Gunnell, Oliver and Peters 56 ) that showed an inverse relationship between lycopene intake or serum concentrations to the molar ratio IGF-1:IGFBP-3; three studies showed no association with the molar ratio (Supplementary Table S1). NHANES III is the first study that examined the molar ratio and its associations with serum concentrations of β-carotene and α-carotene.
Methodological considerations
This summary of study results shows that a diet rich in carotenoids, vegetables and fruits may influence the IGF system and indirectly cancer risk, but the results were diverse. Study design, study population, and use of circulating concentration of carotenoids or dietary intake may influence the results of a study.
Seven of these previous twenty studies were intervention studies; all others were cross-sectional studies. Intervention studies are considered to provide the most reliable evidence in epidemiological research( Reference Hennekens and Buring 57 ). However, results of the intervention studies did not differ from cross-sectional studies (see Supplementary Table S1).
The majority of studies were conducted in Europe and in the USA; only a few were conducted in Japan and in Israel. In a Japanese study( Reference Suzuki, Ito and Hashimoto 6 , Reference Maruyama, Iso and Ito 46 ) both IGF-1 and IGFBP-3 concentrations were positively associated with fruit consumption( Reference Walfisch, Walfisch and Kirilov 44 ); in a second Japanese study( Reference Chew and Park 10 ) lutein/zeaxanthin and IGFBP-3 were inversely associated, and there were no significant results in studies from Europe or the USA. For other carotenoid subgroups and vegetables, we found no differences between European, Asian or US studies.
Of the twenty studies reviewed above, most assessed nutrient intake by FFQ, whereas eight studies measured carotenoid concentration in blood samples. Studies that assessed dietary lycopene intake had similarly inconsistent results as studies that examined circulating lycopene (Supplementary Table S1). Although several studies reported no associations of IGF-1 or IGFBP-3 with either dietary intake or circulating carotenoids (Supplementary Table S1), positive associations with IGF-1 were found for intake of carotenoids( Reference Maruyama, Iso and Ito 46 ) and α-carotene( Reference Graydon, Young and Yarnell 47 ) as well as α- and β-carotene concentration( Reference Suzuki, Ito and Hashimoto 6 ). Negative associations with IGF-1 were found for intake of β-carotene( Reference Norat, Dossus and Rinaldi 45 ). One study reported an inverse association with IGFBP-3 for lutein/zeaxanthin concentration( Reference Suzuki, Ito and Hashimoto 6 ). In conclusion, it appears that the assessment methodology, i.e., measurement of circulating concentrations or dietary intake assessment, has an impact on the results of a study. To further investigate this potential difference, future studies with both measurement methodologies and comparison of the results will help to elucidate these associations.
Individuals who try to eat a healthy diet, such as high in fruits, vegetables or fish, but low in red meat, sugar or salt( Reference Nishida, Uauy and Kumanyika 58 ), are likely to lead a healthy lifestyle in general. The inability to distinguish the effect of diet from that of other lifestyle factors may pose a threat to the validity of diet–disease associations observed in epidemiological studies( Reference Michels 59 ). It has been suggested that carotenoids could act as surrogate markers of a diet high in fruits and vegetables( Reference Mayne 60 ), given that high circulating levels are indeed due to high fruit and vegetable consumption rather than supplement intake. Therefore, we investigated the impact of intake of vegetables and fruits on the IGF system in this study as well. Previously, three studies( Reference Norat, Dossus and Rinaldi 45 , Reference Maruyama, Iso and Ito 46 , Reference Gunnell, Oliver and Peters 56 ) observed an inverse relationship between vegetable intake and IGF-1, IGFBP-3 and their molar ratio, whereas a fourth( Reference McGreevy, Hoel and Lipsitz 50 ) showed positive associations for vegetable intake and IGFBP-3 in African-Americans. The results of NHANES III do not support any of these findings. None of our results for vegetable intake was statistically significant, which is consistent with several other studies (Supplementary Table S1). In contrast to vegetables, fruit intake had a positive association with serum concentrations of IGFBP-3 in women in our analysis. Our findings for the intake of fruits are consistent with those of Maruyama et al.( Reference Maruyama, Iso and Ito 46 ), but in contrast to us, they additionally showed positive associations with serum concentrations of IGF-1. Based on the observation that only few associations with the intake of vegetables and fruits but more with the intake of carotenoids were observed, one might conclude that carotenoids do not merely act as surrogate markers of a generally healthy lifestyle. To eliminate confounding, we adjusted for age, race/ethnicity, BMI, cigarette smoking, alcohol consumption and serum total cholesterol concentration.
Strengths and limitations
The study population is a strength of this study as it is based on a large, nationally representative sample of the US population, and therefore the results have greater external validity than studies of more selected populations( Reference Faupel-Badger, Berrigan and Ballard-Barbash 61 ). The composition and size of the study population provided us with the opportunity to stratify our analyses by sex. We were able to confirm prior observations regarding the relationship between some carotenoids and the IGF system and showed some new associations especially for lycopene. Diet had been assessed for a 4-week period prior to the interview. Differences in fruit and vegetable availability changes due to seasonality might have affected circulating carotenoids. Degradation of stored samples can be problematic in studies, in which these samples have long, complex storage histories. IGF-1 and IGFBP-3 were measured 10–16 years after the blood samples were obtained( Reference Berrigan, Potischmann and Dodd 40 ). A study by Yu et al.( Reference Yu, Mistry and Nicar 62 ) has, however, shown that IGF-1 and IGFBP-3 appear to be stable in response to multiple freeze–thaw cycles. A further limitation of this study is that it is cross-sectional and that our results are based on total circulating IGF-1 levels instead of free IGF-1. Finally, we performed a large number of statistical tests and cannot exclude that some of our finding are simply due to chance.
In conclusion, this analysis further supports that the IGF system, with potential influence on several cancers, may be modified through nutrition, especially carotenoids. In NHANES III, positive relationships between serum concentrations of lycopene, β-carotene, α-carotene, β-cryptoxanthin and lutein/zeaxanthin to IGF-1 concentrations were observed, which is, however, in contrast to the expected inverse associations. The positive associations observed for serum α-carotene and lutein/zeaxanthin concentrations and intake of fruits in relation to IGFBP-3 concentrations are more in line with current thinking of the biological mechanism. Based on ours and the observations of other studies, one might conclude that carotenoids may contribute to IGF-1 and IGFBP-3 modulation, but this very likely depends on the presence of other factors, many of which are still unrevealed or unknown. Clearly, the results deserve confirmation by further larger studies, for example by intervention study to evaluate whether increased consumption of carotenoid-rich fruits and vegetables is able to modulate circulating levels of components of the IGF system.
Supplementary material
The supplementary material for this article can be found at http://www.journals.cambridge.org/10.1017/jns.2016.1
Acknowledgements
There were no conflicts of interest.




