Article contents
Surface engineering for phase change heat transfer: A review
Published online by Cambridge University Press: 20 November 2014
Abstract
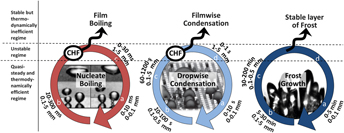
Owing to advances in micro- and nanofabrication methods over the last two decades, the degree of sophistication with which solid surfaces can be engineered today has caused a resurgence of interest in the topic of engineering surfaces for phase change heat transfer. This review aims at bridging the gap between the material sciences and heat transfer communities. It makes the argument that optimum surfaces need to address the specificities of phase change heat transfer in the way that a key matches its lock. This calls for the design and fabrication of adaptive surfaces with multiscale textures and non-uniform wettability.
Among numerous challenges to meet the rising global energy demand in a sustainable manner, improving phase change heat transfer has been at the forefront of engineering research for decades. The high heat transfer rates associated with phase change heat transfer are essential to energy and industry applications; but phase change is also inherently associated with poor thermodynamic efficiency at low heat flux, and violent instabilities at high heat flux. Engineers have tried since the 1930s to fabricate solid surfaces that improve phase change heat transfer. The development of micro and nanotechnologies has made feasible the high-resolution control of surface texture and chemistry over length scales ranging from molecular levels to centimeters. This paper reviews the fabrication techniques available for metallic and silicon-based surfaces, considering sintered and polymeric coatings. The influence of such surfaces in multiphase processes of high practical interest, e.g., boiling, condensation, freezing, and the associated physical phenomena are reviewed. The case is made that while engineers are in principle able to manufacture surfaces with optimum nucleation or thermofluid transport characteristics, more theoretical and experimental efforts are needed to guide the design and cost-effective fabrication of surfaces that not only satisfy the existing technological needs, but also catalyze new discoveries.
- Type
- Review
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 297
- Cited by


