Article contents
The winner takes it all: dominance of Calicophoron daubneyi (Digenea: Paramphistomidae) among flukes in Central European beef cattle
Published online by Cambridge University Press: 24 February 2022
Abstract
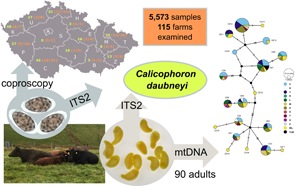
In Europe, paramphistomosis caused by Paramphistomum spp. was historically regarded as being of minor importance. However, Calicophoron daubneyi has recently been recognized as an emerging pathogen in Europe due to its increasing prevalence and negative impact on livestock production. In search for paramphistomid flukes, 5573 beef cattle fecal samples from 115 farms across the whole Czech Republic were examined from March 2019 to June 2021. The eggs of paramphistomid flukes were identified in 29.9% of samples. Internal transcribed spacer 2 sequences from 90 adult flukes and 125 fecal samples collected across Czech Republic confirmed C. daubneyi infection in the Czech beef cattle. Ninety mitochondrial DNA sequences obtained from adult C. daubneyi specimens revealed 13 individual haplotypes, two of them recorded for the first time. Although C. daubneyi is a new parasite in beef cattle herds in the Czechia, it clearly dominates the parasitological findings in the country's beef cattle. The common occurrence of C. daubneyi in most of the beef cattle herds indicates environmental conditions suitable also for the life cycle of Fasciola hepatica and risk of its emergence.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
Footnotes
These authors contributed equally.
References
- 3
- Cited by


