- 25OHD
-
25-hydroxyvitamin D
- 1,25(OH)2D
-
1,25-dihydroxyvitamin D
- DBP
-
vitamin D binding protein
- DC
-
dendritic cell
- DEFB4
-
β-defensin 2
- Gc
-
group-specific component
- IBD
-
inflammatory bowel disease
- NOD2
-
non-obese diabetic 2
- PTH
-
parathyroid hormone
- TB
-
tuberculosis
- Treg
-
regulatory T-cells
- Th1
-
type 1 T-helper
- Th2
-
type 2 T-helper
- TLR
-
Toll-like receptor
- VDR
-
vitamin D receptor
- VDRE
-
vitamin D response element
In 2008, Time magazine listed the ‘benefits of vitamin D’ as one of its top 10 medical breakthroughs for the previous year. Popular recognition such as this reflects the sea change in vitamin D physiology that has taken place over the last 5 years. Two pivotal concepts are central to our new perspective on vitamin D. The first stems from data suggesting that sub-optimal vitamin D status or vitamin D insufficiency is a prevalent health problem across the globe( Reference Holick 1 ). For many years, vitamin D status was broadly defined by whether or not the patient in question presented with rachitic bone disease (osteomalacia in adults). Using this guideline, serum levels of 25-hydroxyvitamin D (25OHD) <8 ng/ml (20 nm) were considered to represent vitamin D deficiency, with higher concentrations being viewed as ‘normal’. Based on these parameters the normal range for vitamin D status in adults was 8–30 ng/ml (20–75 nm). However, more recent studies have shown that classical physiological targets for vitamin D, circulating levels of parathyroid hormone (PTH)( Reference Chapuy, Preziosi and Maamer 2 ), and intestinal Ca uptake( Reference Heaney, Dowell and Hale 3 ), continue to show correlation with serum 25OHD at levels as high as 30 ng/ml (75 nm). It has therefore been concluded that optimal serum 25OHD status is much higher than previously thought, with target concentrations of 30–32 ng/ml (75–80 nm) suggested as optimal( Reference Holick 4 ). As a consequence of this new perspective on adequate vitamin D levels, it has been suggested that sub-optimal vitamin D status, vitamin D insufficiency, is much more common than previously thought( Reference Mithal, Wahl and Bonjour 5 , Reference Dawson-Hughes, Heaney and Holick 6 ).
The second research development that has redefined our perspective on vitamin D concerns the physiological impact of vitamin D insufficiency. Given the classical actions of vitamin D on Ca homoeostasis and bone metabolism, it is likely that vitamin D insufficiency will exert some effects on the skeleton, although these may not be identical to the rachitic bone disease observed with classical vitamin D deficiency( Reference Holick 1 ). However, recent studies have focused on the potential impact of impaired vitamin D status with respect to so-called ‘non-classical’ effects of vitamin D. These include anticancer( Reference Spina, Tangpricha and Uskokovic 7 ) and cardiovascular actions( Reference Zittermann 8 ), but prominent reports have also explored the association between vitamin D and the immune system( Reference Adams and Hewison 9 , Reference Adams and Hewison 10 ). The current review will focus specifically on the link between vitamin D and the immune system, with specific reference to the mechanisms by which variations in vitamin D status may play a pivotal role in defining specific types of immune response. The review will also describe the key health implications associated with vitamin D and human immunity, and the potential benefits this may offer when considering supplemental or therapeutic use of vitamin D.
Vitamin D physiology: classical and non-classical actions
Human subjects obtain most of their vitamin D through the action of sunlight on skin, with 7-dehydrocholesterol being converted photolytically to parental vitamin D in the epidermis. The vitamin D produced in the skin then undergoes sequential metabolic conversions. Firstly, in the liver to form 25OHD the main circulating form of vitamin D. The predominant enzyme involved in this 25-hydroxylation reaction has yet to be definitively identified but is likely to be the cytochrome P450, CYP2R1( Reference Schuster 11 ). Activation of 25OHD to the hormonal form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D) is then catalysed by the enzyme 25OHD-1α-hydroxylase (CYP27B1), which is located primarily in the proximal tubules of the kidney( Reference Schuster 11 , Reference Zehnder, Bland and Walker 12 ). In classical vitamin D physiology, the 1,25(OH)2D produced by the kidneys acts in an endocrine fashion to help regulate mineral homoeostasis and bone metabolism (Fig. 1). Under conditions of low extracellular Ca, Ca-sensing receptors on parathyroid cells signal to increase the secretion of PTH by the parathyroid glands. The resulting rise in serum PTH up-regulates transcription of CYP27B1 in the proximal tubules leading to increased synthesis of active 1,25(OH)2D. This activity is very sensitively regulated via two key mechanisms. The first involves fibroblast growth factor 23, which is closely involved in the regulation of phosphate/Ca metabolism( Reference Liu and Quarles 13 – Reference Yoshiko, Wang and Minamizaki 15 ). Fibroblast growth factor 23 acts mainly as a phosphaturic factor by inhibiting the expression of sodium-phosphate co-transporters in proximal tubular cells( Reference Fukumoto 16 ), but it also suppresses production of 1,25(OH)2D in the kidneys by inhibiting expression of CYP27B1, while stimulating the catabolic enzyme vitamin D-24 hydroxylase (CYP24A1)( Reference Razzaque 17 ). The latter is unique in the steroidogenic world in that it appears to function primarily as a ‘feedback’ control enzyme, limiting the tissue production of active 1,25(OH)2D( Reference Omdahl 18 ).
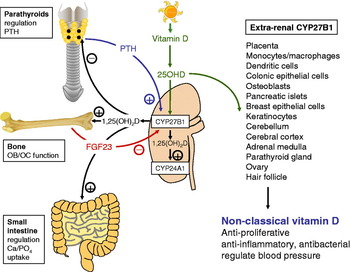
Fig. 1. Renal and extra-renal metabolism of vitamin D. Schematic representation showing key pathways associated with the metabolism and action of vitamin D in normal renal physiology and in extra-renal tissues. The vitamin D-activating enzyme 25-hydroxyvitamin D (25OHD)-1α-hydroxylase (CYP27B1) is expressed in the kidney proximal tubules. Renal CYP27B1 is induced by parathyroid hormone (PTH), and converts 25OHD to 1,25-dihydroxyvitamin D (1,25(OH)2D). The latter is released into the blood stream and also induces renal vitamin D-24-hydroxylase (CYP24A1) activity, leading to feedback synthesis of the less active metabolites, 1,24,25-trihydroxyvitamin D (1,24,25(OH)3D) and 24,25-dihydroxyvitamin D (24,25(OH)2D). Raised serum 1,25(OH)2D acts on distal target organs to: suppress synthesis of PTH by the parathyroid glands; modulate bone-forming osteoblasts (OB) and bone-resorbing osteoclasts (OC) in the skeleton; enhance phosphate and Ca uptake in the intestine. 1,25(OH)2D also stimulates expression of fibroblast growth factor 23 (FGF23), which suppresses renal CYP27B1 activity. Documented extra-renal sites for expression of CYP27B1 are shown, along with putative actions of locally synthesised 1,25(OH)2D within these tissues.
After synthesis in the kidney, 1,25(OH)2D is released into the general circulation and can then act on peripheral tissues. Target cell responses to 1,25(OH)2D are dependent on expression of the intracellular vitamin D receptor (VDR), a member of the nuclear receptor superfamily( Reference Haussler, Haussler and Bartik 19 ). When bound to 1,25(OH)2D the VDR acts as a transcription factor by targeting vitamin D response element (VDRE) DNA motifs within gene promoters( Reference Jurutka, Bartik and Whitfield 20 ). The most well-recognised targets for VDR-mediated regulation of transcription include genes associated with Ca and phosphate uptake in the gastrointestinal tract, and those involved in the regulation of bone turnover in the skeleton( Reference Haussler, Haussler and Bartik 19 , Reference Jurutka, Bartik and Whitfield 20 ). VDR-mediated responses also provide another level of feedback control for the vitamin D system, with serum 1,25(OH)2D acting to negatively regulate the production of PTH by the parathyroid glands( Reference Jurutka, Bartik and Whitfield 20 ). In addition to these classical actions, it has become increasingly clear that the same 1,25(OH)2D–VDR complex can act to regulate expression of target genes not immediately involved in mineral homoeostasis and bone metabolism. Prominent ‘non-classical’ responses to 1,25(OH)2D include anti-proliferative/anticancer effects( Reference Spina, Tangpricha and Uskokovic 7 , Reference Holick 21 ), as well as effects on hypertension( Reference Razzaque 17 , Reference Zhou, Lu and Cao 22 , Reference Li, Kong and Wei 23 ) and immunomodulation( Reference Adams and Hewison 10 , Reference Hewison 24 , Reference Hewison 25 ). A central feature of many of these non-classical actions of vitamin D is that, unlike effects on the skeleton, gut or parathyroids glands, the synthesis of active 1,25(OH)2D appears to occur in a cell-specific manner, with CYP27B1 being expressed by many extra-renal tissues.
Extra-renal synthesis of 1,25-dihydroxyvitamin D
Extra-renal synthesis of 1,25(OH)2D was initially identified in studies of patients with the granulomatous disease sarcoidosis, where macrophages from disease-affected tissues were shown to act as an extra-renal source of CYP27B1( Reference Adams and Gacad 26 ). In this instance, the localised production of 1,25(OH)2D in peripheral tissues affected is sufficient to spill-over into the general circulation and, in some instances, promotes dysregulation of Ca homoeostasis( Reference Papapoulos, Clemens and Fraher 27 ). Subsequent studies have shown that macrophage synthesis of 1,25(OH)2D is common to granulomatous diseases in general, as well as several types of tumour involving significant macrophage infiltration( Reference Hewison, Burke and Evans 28 ). However, expression of CYP27B1 has also been reported for other extra-renal tissues in the absence of any disease( Reference Zehnder, Bland and Williams 29 ).
Historically, the placenta was one of the first extra-renal tissues shown to be capable of synthesising 1,25(OH)2D, with activation of 25OHD being detectable in both maternal decidua and fetal trophoblast( Reference Gray, Lester and Lorenc 30 , Reference Weisman, Harell and Edelstein 31 ). Since then studies of the spatio-temporal organisation of placental CYP27B1 across gestation, has shown that the enzyme is induced early in pregnancy in both decidua and trophoblastic cells but then declines in the third trimester of pregnancy( Reference Zehnder, Evans and Kilby 32 , Reference Evans, Bulmer and Kilby 33 ). Expression of the VDR is also induced in parallel with CYP27B1, consistent with a localised function vitamin D in the placenta, with 1,25(OH)2D synthesised by decidual or trophoblastic cells acting in an autocrine or paracrine fashion( Reference Evans, Bulmer and Kilby 33 ). This mechanism is therefore similar to that conventionally described for expression of CYP27B1 and VDR within cells from the immune system( Reference Adams and Hewison 10 ). The importance of decidual/trophoblast expression of CYP27B1 as an extra-renal feature of the vitamin D system during pregnancy is emphasised by studies of the CYP27B1 knockout mouse. In this animal model, the CYP27B1 gene is replaced with a β-galactosidase reporter construct linked to the endogenous gene promoter for CYP27B1. As a result, transcription of CYP27B1 can be visualised in tissues from the knockout mouse simply by staining for β-galactosidase activity( Reference Vanhooke, Prahl and Kimmel-Jehan 34 ). Using this approach, it was possible to confirm expression of CYP27B1 in classical sites of 1,25(OH)2D production, such as the kidney, but transcription of the enzyme was also strongly detected in the placenta( Reference Vanhooke, Prahl and Kimmel-Jehan 34 ).
The capacity for efficient synthesis of 1,25(OH)2D is further enhanced by studies showing that the vitamin D catabolic enzyme CYP24A1 is poorly expressed in the placenta during early stages of gestation( Reference Evans, Bulmer and Kilby 33 ). The explanation for this appears to be that CYP24A1 gene is highly methylated in the placenta, resulting in tissue-specific silencing of its transcription( Reference Novakovic, Sibson and Ng 35 ). This effect appears to be very selective, and suggests that the placenta is one of the few tissues in which feedback regulation of 1,25(OH)2D is absent( Reference Novakovic, Sibson and Ng 35 ). The net effect of enhanced expression of CYP27B1 in proximity to low or absent CYP24A1 activity is likely to be enhance concentrations of 1,25(OH)2D in the placenta. It is possible that these elevated levels of 1,25(OH)2D will be sufficient to spill-over into the fetal or maternal circulation. This may provide a mechanism for the increased serum levels of 1,25(OH)2D that are characteristic of pregnant women( Reference Kovacs and Kronenberg 36 ). However, current studies suggest that placental CYP27B1 activity also plays a pivotal role in mediating localised responses to vitamin D. In particular, it has been suggested that expression of CYP27B1 in the placenta is crucial to antibacterial and anti-inflammatory responses at the fetal–maternal interface( Reference Evans, Bulmer and Kilby 33 ).
The placenta provides an excellent example of the potential importance of extra-renal 1,25(OH)2D production to normal physiology. However, expression of VDR and CYP27B1 has been reported for many other tissues that can be broadly termed ‘barrier sites’( Reference Jones, Strugnell and DeLuca 37 , Reference Townsend, Evans and Campbell 38 ), indicating that localised responses to vitamin D may be a key feature of these tissues (see Fig. 1). These include the skin, lungs and colon where the function of localised synthesised 1,25(OH)2D does not appear to be directly linked to classical vitamin D endocrinology. Instead, attention has turned to the possible impact of CYP27B1 and VDR components of ‘non-classical’ responses to vitamin D. As illustrated in Fig. 1, this includes anti-proliferative/anticancer effects( Reference Peterlik and Cross 39 , Reference Cross, Kallay and Farhan 40 ), as well as potential actions on the regulation of blood pressure( Reference Li and Batuman 41 ). In addition, much recent attention has focused on the proposed role of vitamin D as an immunomodulatory factor and this is outlined in further detail in the following sections.
Vitamin D and innate antibacterial immunity
It is now more than a quarter of a century since a study was published showing that 1,25(OH)2D potently suppressed proliferation of the infectious pathogen Mycobacterium tuberculosis (M. tuberculosis) in human monocytes( Reference Rook, Steele and Fraher 42 ). At the time, the physiological significance of this was unclear. It was known that patients with tuberculosis (TB) often presented with over-production of 1,25(OH)2D( Reference Epstein, Stern and Bell 43 , Reference Bell, Shary and Shaw 44 ) in a similar fashion to that described earlier for sarcoidosis. However, this was not initially linked to the ability of monocyte CYP27B1 activity to support intracrine killing of M. tuberculosis. Rather it was assumed that therapeutic administration of 1,25(OH)2D or synthetic non-calcaemic analogues of 1,25(OH)2D would provide the most effective conduit for translational use of vitamin D in patients with TB. Surprisingly, it was not until 2006 that this issue was resolved in a series of studies documenting the induction of CYP27B1 in human monocytes treated with immunogens corresponding to M. tuberculosis. Data by Liu et al. showed for the first time that localised synthesis of 1,25(OH)2D by monocytes was an integral part of the normal innate immune function of these cells. Gene array analyses showed that macrophage expression of CYP27B1 and VDR was induced following activation of Toll-like receptor (TLR) 2/1, a pathogen recognition receptor for Gram-positive bacteria and M. tuberculosis( Reference Liu, Stenger and Li 45 ). These observations were consistent with a localised, intracrine system for vitamin D responses in M. tuberculosis-challenged monocytes, and this was confirmed by subsequent studies in which TLR2/1-activated cells were treated with 25OHD. Under these conditions, the resulting locally synthesised 1,25(OH)2D acted to modulate expression of VDR target genes such as CYP24A1. However, intracrine synthesis of 1,25(OH)2D also induced expression of the gene for cathelicidin (LL-37), which encodes a protein known to be involved in promoting intracellular killing of bacteria( Reference Zaiou and Gallo 46 , Reference Zanetti 47 ). Earlier studies indicated that transcription of LL-37 is stimulated in a direct fashion by the 1,25(OH)2D–VDR complex( Reference Wang, Nestel and Bourdeau 48 ) acting via a specific VDRE within the LL-37 gene promoter( Reference Gombart, Borregaard and Koeffler 49 ). Interestingly, this VDRE appears to be specific for primates, and vitamin D does not appear to induce expression of LL-37 in other lower mammals such as mice( Reference Gombart, Borregaard and Koeffler 49 , Reference Gombart, Saito and Koeffler 50 ).
The most notable observation from studies of the intracrine induction of monocyte LL-37 is that this response led to enhanced bacterial killing simply by increasing levels of the precursor form of vitamin D, 25OHD. Consequently, it was proposed that simple variations in vitamin D status could enhance or impair monocyte innate immune responses to infection. This was illustrated by studies showing that monocytes cultured in medium supplemented with serum from vitamin D-insufficient donors produced lower levels of LL-37 following TLR2/1 activation when compared with cells cultured in serum from vitamin D-sufficient donors( Reference Liu, Stenger and Li 45 ). In a similar fashion, serum from vitamin D insufficient subjects supported higher levels of TLR2/1-induced LL-37 following in vivo supplementation with vitamin D( Reference Adams, Ren and Liu 51 ). The overall conclusion from these observations was that vitamin D is an important component of antibacterial activity in monocytes. As such, decreased availability of serum 25OHD due to vitamin D insufficiency has the potential to cause impaired innate immune response to infection.
Since these initial studies, the intracrine model for vitamin D-mediated antibacterial function in monocytes has been expanded to include other mechanisms that further facilitate the immune activity of vitamin D (see Fig. 2). For example, it is now clear that LL-37 is not the only antibacterial target for vitamin D in monocytes. The gene promoter for another antibacterial protein β-defensin 2 (DEFB4) is known to contain VDRE in a similar fashion to LL-37( Reference Wang, Nestel and Bourdeau 48 ), but initially did not appear to be stimulated by 1,25(OH)2D( Reference Liu, Stenger and Li 45 ). However, more recent data have demonstrated 1,25(OH)2D–VDR induction of DEFB4 in conjunction with activation of another transcription factor, NF-κB. Induction of NF-κB following treatment of monocytes with cytokines such as IL-1β( Reference Liu, Schenk and Walker 52 ) or as a consequence of signalling via the intracellular pathogen recognition receptor non-obese diabetic 2 (NOD2)( Reference Wang, Dabbas and Laperriere 53 ), have been shown to enhance 1,25(OH)2D-mediated induction of DEFB4. Vitamin D has also been shown to promote the environment in which monocytes carry out bacterial killing. Monocytes treated with 1,25(OH)2D show increased levels of autophagy, an intracellular mechanism known to be essential for the general cytoplasmic homoeostasis in eukaryotes( Reference Klionsky and Emr 54 ). Autophagy and formation of associated autophagosomes are also known to be important as a mechanism for intracellular isolation of pathogens and their subsequent eradication by antibacterial proteins( Reference Levine and Deretic 55 ). Vitamin D-mediated induction of autophagosomes in monocytes is associated with enhanced capacity for intracellular killing of M. tuberculosis, but appears to be mediated indirectly via increased transcription of LL-37( Reference Yuk, Shin and Lee 56 ). Subsequent studies have shown that, consistent with the initial studies of intracrine M. tuberculosis induction of LL-37, TLR2/1-mediated induction of autophagy appears to involve induction of 25OHD metabolism via CYP27B1( Reference Shin, Yuk and Lee 57 ), suggesting that this mechanism will also be highly influenced by changes in vitamin D status.
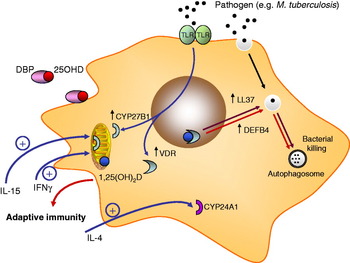
Fig. 2. Vitamin D and monocyte antibacterial activity. Monocyte Toll-like receptor (TLR2) signalling results in transcriptional induction of the vitamin D receptor (VDR) and 1α-hydroxyase (CYP27B1). Circulating 25-hydroxyvitamin D (25OHD) bound to serum vitamin D binding protein (DBP) enters monocytes and is converted to 1,25-dihydroxyvitamin D (1,25(OH)2D) by mitochondrial CYP27B1. VDR-bound 1,25(OH)2D is then able to act as a transcriptional factor, inducing expression of cathelicidin (LL-37) and β-defensin 2 (DEFB4) (the latter in conjunction with NF-κB). 1,25(OH)2D-induced LL-37 promotes autophagy (LC3 expression) and the formation of autophagosomes. Expression of CYP27B1 is also stimulated by the cytokines IL-15 and interferon γ (IFNγ). Conversely, monocyte synthesis of 1,25(OH)2D is suppressed by IL-4, which acts to promote catabolic CYP24A1 activity. 1,25(OH)2D produced by monocytes may also act on other immune cells, notably those from the adaptive immune system.
Induction of antibacterial activity by vitamin D metabolites is not restricted to monocytes and macrophages. Vitamin D-mediated induction of LL-37 has been reported for a variety of cell types including bronchial epithelial cells( Reference Yim, Dhawan and Ragunath 58 ), myeloid cell lines( Reference Gombart, Borregaard and Koeffler 49 ), decidual( Reference Evans, Nguyen and Chan 59 ) and trophoblastic cells of the placenta( Reference Liu, Kaplan and Low 60 ). However, this response is not universal( Reference Schauber, Dorschner and Yamasaki 61 ), and in those cells that do show induction of LL-37 by vitamin D, the precise mechanism may be different to that shown in Fig. 2. For example, human keratinocytes have relatively low expression of TLR2 and are therefore less sensitive to ligands for this pathogen recognition receptor( Reference Schauber, Dorschner and Coda 62 ). In this setting, other tissue-specific factors such as transforming growth factor-β can act to compensate. Transforming growth factor-β potently stimulates CYP27B1 expression in keratinocytes, leading to increased levels of 1,25(OH)2D in the skin. This, in turn, stimulates TLR expression, leading to enhanced sensitivity to TLR2 ligands leading ultimately to further elevation of epidermal CYP27B1 and enhanced vitamin D-mediated production of antimicrobial LL-37( Reference Schauber, Dorschner and Coda 62 ). Because transforming growth factor-β is released in the skin following epidermal wounding, it has been suggested that vitamin D-induced LL-37 may provide a mechanism for the prevention of infection following wounding. Another recently reported mechanism that appears to enhance vitamin D-mediated antibacterial activity is signalling via the intracellular pathogen-recognition receptor NOD2. Expression of NOD2 is potently induced by 1,25(OH)2D in a variety of cell types, enhancing cell sensitivity to the NOD2 ligand muramyl dipeptide, a product of Gram-positive and Gram-negative bacteria( Reference Wang, Dabbas and Laperriere 63 ). NOD2 activates NF-κB, and this has been shown to potentiate vitamin D-mediated transcription of LL-37 and DEFB4( Reference Wang, Dabbas and Laperriere 63 ). Similar NF-κB-potentiation of vitamin D-induced DEFB4 has also been described for IL-1β, suggesting that cytokines from other parts of the normal immune system may act to fine tune innate antibacterial responses to vitamin D.
Vitamin D, antigen presentation and innate immunity
Effective management of infection not only involves adequate innate immune management of intracellular bacteria but also requires appropriate adaptive or acquired immune activity. At the interface between these two mechanisms are antigen-presenting cells, which present bacterial antigens to cells from the adaptive immune system such as T-lymphocytes (T-cells). Macrophages are able to fulfil this function, but antigen presentation is more effectively executed by dendritic cells (DC). It was recognised many years ago that DC isolated from lymphoid tissue express VDR( Reference Brennan, Katz and Nunn 64 ), indicating that they were a likely target for vitamin D-mediated immunoregulation. This was confirmed by studies showing that treatment with 1,25(OH)2D suppressed DC maturation and thereby promoted a tolerogenic phenotype( Reference Penna and Adorini 65 , Reference Adorini, Penna and Giarratana 66 ). This effect was more pronounced in myeloid DC relative to plasmacytoid DC, despite both subsets expressing similar levels of VDR( Reference Penna, Amuchastegui and Giarratana 67 ). Under steady state conditions myeloid DC are more active at priming naive T-cell responses. By contrast plasmacytoid DC exhibit more tolerogenic, immunosuppressive properties. Consequently, 1,25(OH)2D appears to fulfil a more tolerogenic function by suppressing activity of myeloid DC, while leaving the already tolerogenic plasmacytoid DC unaffected.
DC share the same cell lineage as monocytes and macrophages, and show the similar patterns of VDR and CYP27B1 expression( Reference Hewison, Freeman and Hughes 68 ). Consequently, studies using monocyte-derived DC showed that both 1,25(OH)2D and 25OHD are able to suppress the maturation and function of these cells( Reference Hewison, Freeman and Hughes 68 ). Differentiation of DC towards a mature, antigen-presenting phenotype, leads to increased expression of CYP27B1 but with a reciprocal suppression of VDR levels( Reference Hewison, Freeman and Hughes 68 ). It therefore seems likely that any 1,25(OH)2D produced by mature DC will not act in an intracrine fashion due to low VDR levels. Instead a paracrine mechanism is more likely with VDR-rich immature DC responding to 1,25(OH)2D produced by VDR-depleted mature DC. This mechanism may be important because it enables some DC to mature thereby facilitating activation of normal immune responses, while preventing exaggeration of this response and possible pathological effects. The importance of vitamin D as a modulator of DC function is supported by studies of VDR and CYP27B1 gene knockout mice, in which these animals present with lymphatic abnormalities consistent with increased numbers of mature DC( Reference Griffin, Lutz and Phan 69 , Reference Panda, Miao and Tremblay 70 ) and aberrant DC trafficking( Reference Enioutina, Bareyan and Daynes 71 ).
Vitamin D, innate immunity and human disease
Irrespective of recent developments in the intracrinology of innate immunity, there is an historical precedence linking vitamin D and infectious disease. In 1903, Niels Finsen received the Nobel Prize for Medicine after he demonstrated that he could cure Lupus Vulgaris (the epidermal form of TB) with exposure to light from an electric arc lamp. In a similar fashion, cod liver oil, a rich source of dietary vitamin D was also used as a treatment for TB( Reference Grad 72 ). With this in mind, and the recent studies showing TLR2/1 activation of monocyte vitamin D metabolism, it is not surprising that translation studies have explored further the link between vitamin D and the disease TB. Epidemiology has shown that vitamin D-insufficiency (serum 25OHD <75 nm) is associated with increased incidence of TB( Reference Wilkinson, Llewelyn and Toossi 73 – Reference Wejse, Gomes and Rabna 76 ). Several clinical trials of vitamin supplementation have also been reported with varying success( Reference Wejse, Gomes and Rabna 76 – Reference Nursyam, Amin and Rumende 78 ). The most recent supplementation study using 4×2.5 mg vitamin D was successful in raising serum levels of 25OHD in TB patients, but showed no overall difference in sputum conversion time between treatment and placebo groups( Reference Martineau, Timms and Bothamley 79 ). However, the authors did show a significant improvement in sputum conversion in a specific subset of TB patients with a Taq1 single nucleotide polymorphism within the VDR gene( Reference Martineau, Timms and Bothamley 79 ). Thus inherited factors may influence responses to vitamin D supplementation and this facet of vitamin D physiology. Another example of this is provided by the gene for vitamin D binding protein (DBP), the main serum carrier of vitamin D metabolites. Recent studies by our group have shown that the ability of 25OHD or 1,25(OH)2D to stimulate antibacterial activity in monocytes is affected by serum levels of DBP and its binding affinity for vitamin D metabolites( Reference Chun, Lauridsen and Suon 80 ). Both of these parameters are influenced by DBP genotype, notably the alleles referred to as group-specific component (Gc)IF, Gc1S and Gc2( Reference Lauridsen, Vestergaard and Hermann 81 , Reference Arnaud and Constans 82 ). Data from our studies suggest that there is greater bioavailability of 25OHD or 1,25(OH)2D in the presence of low affinity forms of DBP such as Gc1S and Gc2( Reference Chun, Lauridsen and Suon 80 ). This observation supports the ‘free hormone hypothesis’ in which steroid hormones are able to passively diffuse across cell membranes when they are not bound to carrier proteins. However, it is important to recognise that the opposite scenario, binding of 25OHD to DBP, is important for classical vitamin D endocrinology. DBP-bound 25OHD is recovered from glomerular filtrates via an endocytic mechanism involving the membrane receptor megalin prior to its conversion to 1,25(OH)2D in the proximal tubules( Reference Nykjaer, Dragun and Walther 83 ).
The link between vitamin D and infection is unlikely to be restricted to TB. Serum levels of 25OHD have been shown to correlate with circulating levels of LL-37 and increased risk of critical illness in patients with sepsis( Reference Jeng, Yamshchikov and Judd 84 ). Low vitamin D status has also been linked to increased infection and mortality in chronic kidney disease( Reference Gombart, Bhan and Borregaard 85 ), and seasonal variations of infections such as influenza, the latter highlighting a potential role for vitamin D in counteracting infection in the upper respiratory tract( Reference Cannell, Vieth and Umhau 86 ). However, it is also important to recognise that the innate immune regulatory effects of vitamin D may not be restricted to infectious disease. For example, vitamin D-deficient mice show suppressed colonic expression of angiogenin-4, an antimicrobial protein produced primarily in Paneth cells which acts to minimise tissue invasion by enteric bacteria( Reference Hooper, Stappenbeck and Hong 87 ). In view of the fact that aberrant innate immune response to enteric bacteria has been postulated to initiate tissue inflammation in some types of inflammatory bowel disease (IBD)( Reference Packey and Sartor 88 ), it is possible to speculate a role for vitamin D in protecting against this disease via the induction of angiogenin-4 antibacterial responses to enteric bacteria within the gastrointestinal tract. Finally, vitamin D may also play a role in promoting innate immune responses to non-living material. Studies using monocytes obtained from patients with Alzheimer's disease have shown that these cells are less able to phagocytose and degrade β-amyloid protein( Reference Masoumi, Goldenson and Ghirmai 89 ). Treatment with 1,25(OH)2D potently enhanced monocyte phagocytosis and degradation of β-amyloid, suggesting a role for vitamin D-mediated immunity in this neurological disorder.
Vitamin D and adaptive immunity
Although much of the recent interest in non-classical vitamin D action has stemmed from studies of monocyte antibacterial activity, it is clear that there are many other links between vitamin D and the immune system. For example, immune responses to pathogens such as M. tuberculosis are not restricted simply to TLR2/1-induced expression of LL-37 or DEFB4, but instead involve other facets of immunity. Promoter–reporter analysis of the transcriptional regulation of CYP27B1 suggests that TLR-mediated induction of the enzyme involves JAK-STAT (Janus kinase-signal transducer and activator of transcription), mitogen-activated protein kinase and NF-κB pathways, but these signalling pathway also synergise with cytokine-mediated induction of CYP27B1( Reference Stoffels, Overbergh and Giulietti 90 , Reference Krutzik, Hewison and Liu 91 ). In particular, recent studies have shown that cytokines from different T-cell subsets exert very specific effects on innate immune responses to vitamin D. Interferon γ, a cytokine produced by type 1 T-helper (Th1) cells potently enhances TLR2/1-induced expression of CYP27B1 and associated bacterial killing( Reference Edfeldt, Liu and Chun 92 ). By contrast IL-4 a cytokine produced by type 2 T-helper (Th2) cells acts to attenuate TLR2/1-activated bacterial killing. However, in this instance, the action of IL-4 was not due to effects on CYP27B1, but instead involved enhanced CYP24A1 activity( Reference Edfeldt, Liu and Chun 92 ). In view of this divergence between effects of Th1 and Th2 cytokines on monocyte 25OHD metabolism, it is interesting to speculate that vitamin D may play a key role at the boundary between the innate and adaptive immune systems. What is certainly clear is the independent of its innate immune activity vitamin D can act as a potent regulator of the adaptive immune system as well( Reference Peelen, Knippenberg and Muris 93 ).
One of the initial observations linking vitamin D with the adaptive immune system was that T-cells and B-lymphocytes (B-cells) express VDR( Reference Bhalla, Amento and Clemens 94 , Reference Provvedini, Tsoukas and Deftos 95 ), with these levels increasing as T- or B-cells proliferate( Reference Nunn, Katz and Barker 96 ). As a consequence, initial studies of the effects of vitamin D on T-cells focused on the ability of 1,25(OH)2D to suppress T-cell proliferation( Reference Nunn, Katz and Barker 96 – Reference Karmali, Hewison and Rayment 98 ). However, subsequent studies showed that vitamin D could also influence the phenotype of T-cells, notably through inhibition of Th1 cells, a subset of CD4+ effector T-cells closely associated with cellular immune responses( Reference Lemire, Archer and Beck 99 ). In concert with this 1,25(OH)2D was also shown to enhance cytokines associated with Th2 cells, a subset of CD4+ T-cells associated with humoral immunity( Reference Overbergh, Decallonne and Waer 100 , Reference Boonstra, Barrat and Crain 101 ). It was therefore suggested that vitamin D could help limit the tissue damage associated with excessive Th1 cellular immune responses by switching T-cells to a Th2 phenotype. Subsequent studies using VDR gene knockout mice have questioned the validity of this hypothesis in that these animals have reduced rather than elevated levels of Th1 cells( Reference O'Kelly, Hisatake and Hisatake 102 ). Thus, although vitamin D appears to promote a Th1 to Th2 shift in vitro, it seems likely that its effects on T-cells in vivo are more complex. More recent reports have shown that in addition to Th1 or Th2 cells, there is a third effector T-cell population termed Th17 cells because of their capacity to synthesise IL-17( Reference Harrington, Mangan and Weaver 103 , Reference Weaver, Hatton and Mangan 104 ). Th17 cells are important for promoting immune responses to some pathogens, but they have also been linked to inflammatory tissue damage( Reference Bettelli, Korn and Kuchroo 105 , Reference Korn, Oukka and Kuchroo 106 ). Treatment of T-cells in vitro with 1,25(OH)2D suppresses Th17 development( Reference Colin, Asmawidjaja and van Hamburg 107 , Reference Palmer, Lee and Maynard 108 ), and inhibits of IL-17 production via a post-transcriptional mechanism( Reference Chang, Chung and Dong 109 ). In a similar fashion, in vivo mouse models of IBD have shown that treatment with 1,25(OH)2D down-regulates expression of IL-17( Reference Daniel, Sartory and Zahn 110 ). By contrast, loss of 1,25(OH)2D in vivo as a result of CYP27B1 gene knockout leads to elevated levels of IL-17( Reference Liu, Nguyen and Chun 111 ).
The adaptive immune effects of vitamin D are not restricted to effector T-cells, and also include actions on suppressor or regulatory T-cells (Treg), a group of CD4+ T-cells known to inhibit the proliferation of other CD4+ T-cells. Treatment of naive CD4+ T-cells with 1,25(OH)2D potently induces the development of Treg( Reference Gorman, Kuritzky and Judge 112 ), and this may exert beneficial effects in autoimmune disease and host–graft rejection( Reference Gregori, Giarratana and Smiroldo 113 – Reference Spach, Nashold and Dittel 115 ). Although, 1,25(OH)2D can stimulate Treg development directly via VDR expression by CD4+ T-cells( Reference Jeffery, Burke and Mura 116 , Reference Urry, Xystrakis and Richards 117 ), it may also act via effects on antigen-presenting cells. Specifically, as outlined earlier, the ability of 1,25(OH)2D to induce an immature DC phenotype will promote tolerogenic Treg activity in CD4+ T-cells( Reference Gregori, Casorati and Amuchastegui 118 – Reference Adorini, Penna and Giarratana 120 ). In view of the fact that DC express CYP27B1 as well as VDR, this indirect mechanism for inducing Treg is also likely to be stimulated by 25OHD, providing a possible link between low serum vitamin D status and impaired Treg activity( Reference Hewison, Burke and Evans 28 ). The overall conclusion from the various studies of T-cell phenotype is that vitamin D acts to maintain a balance between inflammatory Th1/Th17 cells and immunosuppressive Th2/Treg (Fig. 3).

Fig. 3. Vitamin D and T-cell function. Under conditions of vitamin D sufficiency, synthesis of 1,25-dihydroxyvitamin D (1,25(OH)2D) within the immune system acts to maintain a tolerogenic immune response by favouring Th2 and Treg v. Th1 and Th17 cells. Conversely, vitamin D insufficiency will favour a more inflammatory immune response involving Th1/Th17 cells rather than Th2/Treg.
In common with CD4+ effector T-cells, CD8+ cytotoxic T-cells express abundant VDR and are sensitive to cytokine regulation by 1,25(OH)2D( Reference Willheim, Thien and Schrattbauer 121 ), but when compared with CD4+ T-cells they are relatively insensitive to anti-proliferative responses( Reference Veldman, Cantorna and DeLuca 122 , Reference Iho, Iwamoto and Kura 123 ). The physiological relevance of 1,25(OH)2D responses in CD8+ T-cells remains unclear. For example, 1,25(OH)2D can protect against the mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis ( Reference Cantorna, Hayes and DeLuca 124 ). However, this effect does not appear to require the presence of CD8+ cells despite the fact that these cells have been implicated in multiple sclerosis and experimental autoimmune encephalomyelitis disease pathophysiology( Reference Meehan and DeLuca 125 ). Effects of vitamin D on CD8+ T-cells may be subset-specific. The CD8 molecule on T-cells can be expressed as either an α–β heterodimer or as an α–α homodimer, and the latter appear to be influenced by vitamin D. Studies using the VDR gene knockout mouse have shown aberrant gut migration of CD8+ α–α cells and this appears to be linked to increased risk of IBD in these animals( Reference Yu, Bruce and Froicu 126 ). This is similar to the positive effect of 1,25(OH)2D on epidermal T-cell homing( Reference Sigmundsdottir, Pan and Debes 127 ), but contrasts its negative effects on T-cell homing to lymph nodes( Reference Topilski, Flaishon and Naveh 128 ).
Early studies demonstrated that 1,25(OH)2D could also act on VDR-expressing B-cells to suppress cells proliferation( Reference Shiozawa, Shiozawa and Shimizu 129 ) and Ig production( Reference Provvedini, Tsoukas and Deftos 130 ). More recent reports confirmed these effects and also showed that 1,25(OH)2D can also inhibit the differentiation of plasma cells and class-switched memory cells( Reference Chen, Sims and Chen 131 ), highlighting a potential role for vitamin D in B-cell-related disorders such as systemic lupus erythamtosus. Interestingly, this study also demonstrated B-cell expression of CYP27B1, indicating that B-cells may be capable of autocrine/intracrine responses to vitamin D( Reference Chen, Sims and Chen 131 ). This mechanism may be common to lymphocytes in general as CYP27B1 expression has also been reported in T-cells( Reference Sigmundsdottir, Pan and Debes 127 ).
Vitamin D, adaptive immunity and human disease
Although the adaptive immune system is essential for much of the innate immune activity outlined in previous sections, it clear that vitamin D may also be linked to diseases more closely associated with T- and B-cell function. In particular, increasing numbers of studies have linked vitamin D insufficiency to increased risk or severity of autoimmune disease( Reference Holick and Chen 132 , Reference Adorini and Penna 133 ). Low vitamin D status has been linked to type 1 diabetes( Reference Mathieu, Gysemans and Giulietti 134 , Reference Littorin, Blom and Scholin 135 ), and supplementation with vitamin D has been reported to protect against this disease( Reference Harris 136 ). In a similar fashion, analysis of the NOD mouse, an animal model for type 1 diabetes, has shown increased disease severity under conditions of dietary vitamin D restriction( Reference Giulietti, Gysemans and Stoffels 137 ). Another strand of evidence linking vitamin D with type 1 diabetes is provided by the extensive genetic analyses that have investigated the physiological impact of polymorphic variations in the genes for various components of the vitamin D metabolic and signalling system. Specific VDR gene haplotypes appear to protect against diabetes( Reference Ramos-Lopez, Jansen and Ivaskevicius 138 ), and polymorphisms in the CYP27B1 gene have also been shown to affect diabetes susceptibility( Reference Bailey, Cooper and Zeitels 139 ).
Other autoimmune diseases linked to vitamin D insufficiency include multiple sclerosis (reviewed in( Reference Raghuwanshi, Joshi and Christakos 140 )). Studies of human multiple sclerosis patients are supported by analysis of the experimental autoimmune encephalomyelitis mouse of multiple sclerosis, which shows increased disease severity under dietary vitamin D restriction( Reference Spach and Hayes 141 ). Therapeutic administration of 1,25(OH)2D to experimental autoimmune encephalomyelitis mice has been shown to protect against disease symptoms( Reference Spach, Pedersen and Nashold 142 , Reference Pedersen, Nashold and Spach 143 ), with this effect involving regulation of cytokine synthesis, notably IL-10 activity, and apoptosis of inflammatory cells( Reference Spach, Nashold and Dittel 115 ). In a similar fashion to type 1 diabetes and multiple sclerosis, epidemiology suggests that patients with Crohn's disease, a form of IBD have decreased serum levels of 25OHD( Reference Vagianos, Bector and McConnell 144 – Reference Pappa, Grand and Gordon 146 ). Likewise, studies using various experimentally induced forms of IBD in mice indicate that 1,25(OH)2D plays a crucial role in the pathophysiology of this disease( Reference Liu, Nguyen and Chun 111 ,147–Reference Froicu, Weaver and Wynn 149 ). Crohn's disease is considered to be an autoimmune disease, with the disease aetiology appearing to be due to aberrant colonic immune responses to enteric bacteria. Intriguingly, current studies have implicated aberrant innate immune handling of enteric microbiota as an initiator of the adaptive immune damage associated with Crohn's disease( Reference Packey and Sartor 88 ). Consequently, it is possible that the effects of vitamin D on IBD may involve both the activation of innate immunity, together with the suppression of adaptive immunity and associated inflammation.
Conclusions
Although the interaction between vitamin D and the immune system has been recognised for almost 30 years, it is only in the last few years that the physiological relevance of vitamin D-mediated immunity has become clear. Studies using human cells and animal models have highlighted potent effects of vitamin D on both innate and adaptive immune responses in a wide variety of tissues. These observations support the overall hypothesis that vitamin D may play a role in promoting elimination of pathogens such as M. tuberculosis, while suppressing the potentially damaging effects of prolonged inflammation. As such, vitamin D has the potential to influence a wide range of immune disorders, notably infectious and autoimmune diseases. At a clinical level, associated studies have expanded functional data to show that vitamin D insufficiency is linked to several common immune health problems.
Many challenges remain. For example, innate antibacterial activity of vitamin D appears to be restricted to primates, which express the promoter VDRE required for vitamin D-mediated transcriptional regulation of antibacterial proteins. This raises the question as to whether or not vitamin D plays a role in innate immunity in mouse models? Some mouse antimicrobial molecules such as angiogenin-4 appear to be influenced by vitamin D( Reference Lagishetty, Misharin and Liu 150 ), but are there other targets? In contrast to the innate immune system, most of the reported actions of vitamin D on adaptive immunity are focused on suppressive actions. However, recent studies suggest that vitamin D may also be involved in directing T-cell activation( Reference von Essen, Kongsbak and Schjerling 151 ). Although this mechanism is considered to be controversial( Reference Smolders, Thewissen and Damoiseaux 152 ), it underlines the exciting new developments that characterise the current interest in vitamin D and the immune system. Perhaps the most important challenge facing vitamin D immunity research is the evolution of clinical studies from observational association analyses to prospective clinical trials. For many diseases, notably autoimmune diseases, this is a huge logistical challenge and is complicated by uncertainty over whether vitamin D can be used as therapy for some diseases or whether it simply acts to protect against the onset of disease.
Acknowledgements
The author declares no conflict of interest.





