Article contents
Carbon-doped titania as a precursor for titanate nanotubes
Published online by Cambridge University Press: 03 April 2018
Abstract
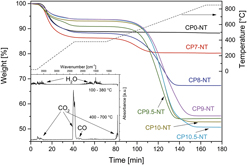
Carbon-doped titania was fabricated via carbothermal treatment in nitrogen–acetylene gas flow and further used as a precursor for multiwalled titanate nanotube (TNT) synthesis via alkaline hydrothermal route. Investigation of the reaction products after hydrothermal treatment of carbon-doped titania using Transmission electron microscopy, X-ray diffraction, and Brunauer–Emmett–Teller method shows the successful formation of TNTs. The presence of carbon was proved although the type of incorporation could not be certified. All samples show approximately the same carbon content before and after hydrothermal treatment. An increasing pretreatment temperature of titania precursor powders yields more secondary products in the nanotube samples, indicating lower reactivity of the titanium oxycarbide phase during hydrothermal treatment. In this study, TNTs with 6 wt% carbon and with the highest specific surface area of 340 m2/g were formed via hydrothermal treatment of carbon-doped titania precursor powder exposed to 700 °C during carbothermal pretreatment.
Keywords
- Type
- Article
- Information
- Journal of Materials Research , Volume 33 , Issue 9: Focus Issue: Porous Carbon and Carbonaceous Materials for Energy Conversion and Storage , 14 May 2018 , pp. 1288 - 1300
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 3
- Cited by



