Vertigo is the sensation of self-motion when no self-motion is occurring or the sensation of distorted self-motion during an otherwise normal head movement. The etiology of vertigo is hard to distinguish. The visual perception, proprioceptive, and vestibular system constitute the “equilibrial triad.” The vestibular system is the primary mechanism in the maintenance of posture balance, and its pathological change is one of the main causes of vertigo. Currently, a sensitive and specific vestibular function test is not widely available in clinics. The video head impulse test (vHIT) is a quick and simple test for the examination of vestibular impairment, with a potentially clinical application.Reference Weber, MacDougall and Halmagyi 1 - Reference Armato, Ferri, Pinzani and Ulmer 4 The test is mainly based on the principle of vestibulo-ocular reflex (VOR), and reflects vestibular function under high-frequency stimulation.Reference Halmagyi and Curthoys 5 , Reference Grossman, Leigh and Bruce 6 Theoretically, the normal value of VOR is 1. We used vHIT to investigate vestibular functions in patients with acute vertigo in an early stage to help with etiological diagnoses. We focused on the study of vestibular neuritis (VN), which is associated with a high morbidity rate and is easily confused with other vertigo.Reference Strupp and Brandt 7 VN primarily manifests as severe vertigo without hearing loss and is associated with peripheral vestibular impairment without involving the cochlea or central nervous system. It is still a diagnosis of exclusion. To distinguish VN from other vestibular syndrome was significant in the emergency department, and we used vHIT to determine if early diagnosis of VN might help patients to receive early treatment, such as glucocorticoids and antiviral agents.Reference Strupp, Zingler and Arbusow 8
Methods
Patients
Patients with dizziness and vertigo symptoms whose vestibular syndrome occurred within a week while without hearing loss were recruited from March 15, 2015, through September 10, 2015, and were selected from the Sir Run Run Shaw Hospital affiliated with the Zhejiang University School of Medicine and Ningbo No. 2 hospital. Grouping was based on a final clinical diagnosis; exclusion would be done before hospital discharge in case of faulty diagnosis at admission. Finally, 33 patients with VN were selected, including 16 males and 17 females ages 28 to 70 years (mean, 53±14.9 years). The total number of other acute vertigo (AV) patients was 96, including 40 males and 56 females ages 26 to 82 years (mean, 53.0±15.1 years). Forty-three patients were diagnosed benign paroxysmal positional vertigo, 17 males and 26 females, ages 26 to 78 years (mean, 48.2±11.5 years). Psychogenic vertigo occurred in six females but no males. Pressure vertigo occurred in one female. Vertigo related to other systemic diseases was found in three males and three females. Paroxysmal vestibular disorders occurred in two females. Migraine-associated vertigo was observed in one female. Drug-induced vertigo was observed in one male. Epileptic vertigo occurred in one male. Posterior circulation infarction was observed in five males and three females. Uncertain vertigo concluded 12 males and 14 females. Normal controls were recruited from staff working at the Sir Run Run Shaw Hospital and Ningbo No.2 hospital, relatives of patients or volunteers, including 20 males and 30 females ages 21 to 81 years (mean, 50.5±12.4 years). No history of tinnitus, deafness, dizziness, or balance impairment was recorded in the controls. No significant difference was observed in the male-to-female ratio or age among the VN and normal control group, AV group (p>0.05). Medical history and physical examination were assessed in all subjects. Severe vision damage with inability to focus on visual targets or impaired eye movement, intracranial surgery, or a history of ototoxic drugs was excluded. Hearing test of pure tone audiometry (PTA) was used in patients identified with possible decreased hearing. Considering the possibility of reduced hearing in the elderly, patients older than age 65 with PTA more than 40 dB,Reference Ventry and Weinstein 9 or less than 65 with PTA more than 25 dB were excluded. The present study was explained to patients and written informed consents were obtained. The protocol was approved by the Ethics Committee of the Sir Run Run Shaw hospital. The study conformed to ethical principles for medical research involving human subjects in accordance with the Declaration of Helsinki.
Diagnosis of VN
Clinical diagnostic criteria of VNReference Mantokoudis, Tehrani and Wozniak 10 were as follows: (1) acute or subacute onset of spontaneous vertigo occurred at least for more than 1 hour, or accompanied with nausea and vomiting; (2) spontaneous horizontal nystagmus, or accompanied with oscillating illusion of vision; (3) new-onset postural or imbalanced gait or accompanied with ataxia; (4) hearing impairment excluded by PTA; (5) no neurological deficit; (6) normal (e.g. changes related to age) or nonspecific abnormal magnetic resonance imaging-diffusion-weighted imaging (after symptoms for 1 to 10 days); (7) exclusion of previous history of vestibular dysfunction or impaired eye movement, acute drug/alcoholic poisoning, and recent head trauma; and (8) follow-up for at least more than 1 week. A final diagnosis was made by more than two neurologists with 10 years’ clinical experience.
vHIT Methods
vHIT was used to examine all the patients and normal controls. An ICS Impulse® instrument of vHIT (GN Otometrics, Denmark) comprises computer analysis, a recording system, and an infrared video camera. The data acquisition system was obtained from a light texture eye mask placed on the patient’s head and a video camera head placed on his or her right eye. During examination, the subject was in the sitting position at 1 m distance and was required to fix his or her gaze horizontally on a visual target. An experimenter stood behind the subject holding the subject’s head. The subject was advised to relax the neck, and a head impulse test (HIT) was performed in each direction of the semicircular canal (SCC). Direction and timing of HIT were irregular and unpredictable. Peak head velocity of the impulses was gradually increased, ranging from 50° to 250°/second (acceleration was 750-5000°/s2, amplitude was 5° to 20°). At least 20 standard HITs were performed at each SCC and recorded by ICS impulse. HITs that did not meet the standard criteria were excluded from the tests. A pair of horizontal (left lateral/right lateral) VOR gains, and two pairs of vertical (left anterior/right posterior, and left posterior/right anterior) VOR gains were measured separately. Movement of a subject’s head and eye during passive HIT was recorded and analyzed. One horizontal pair and two pairs of vertical VOR gains (gain=speed of eye movement/speed of head movement) and their bilateral asymmetry was automatically calculated separately. The final results for each subject were obtained from effective data of more than 15 HIT tests. We set the degree of dispersion (σ=standard deviation) at less than 0.10 in the horizontal plane and less than 0.15 in the vertical plane to ensure accuracy and consistency of the data.
Statistical Analysis
Statistical analysis was performed using SPSS 20 (IBM Corp., Armonk, NY). Measurement of VOR gain and asymmetry data was summarized as mean±standard deviation and verified by normal distribution. Count data such as number of positive or negative results were compared with a two-tailed t test (alpha=0.01).
Results
Results of VOR Gains and Asymmetry in the Normal Control (NC) Group
The VOR gains were infinitely close to 1, particularly in the horizontal semicircular canal. No significant difference was observed in VOR gains and asymmetry among different SCC (p>0.05; Table 1, NC group).
Table 1 Comparison of vHIT VOR gains and asymmetry results within VN group, NC group, and AV group
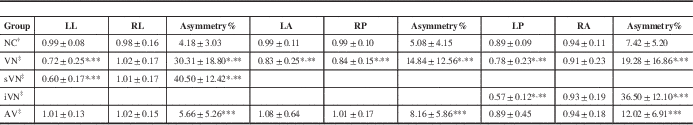
Values are mean gain±standard deviation (n=50 in the NC group, n=33 in the VN group, n=96 in the AV group). The data were analyzed by a two-tailed t test. *p<0.001 VN vs NC groups. **p<0.001 VN vs AV groups. ***p<0.05 VN vs NC groups. The pairs were matched based on sequences if the patients were positioned according to disease condition.
† The VOR gains and asymmetry in the NC group were calculated in LL/RL, LA/RP, and LP/RA pairs.
‡ The VOR gains and asymmetry in the VN group were calculated based on affected lateral/intact lateral, affected anterior/intact posterior, and affected posterior/intact anterior.
Benign paroxysmal positional vertigo patients were the same as the VN group based on niveau (left or right side). Asymmetry was location independent. Except for one case that could not be distinguished, 22 cases involving horizontal SCC function loss were defined as sVN dysfunction, seven cases involving vertical SCC function loss were defined iVN, and three cases involving both were analyzed separately.
iVN, inferior vestibular nerve; LA, left anterior; LL, left lateral; LP, left posterior; RA, right anterior; RL, right lateral; RP, right posterior; sVN, superior vestibular nerve.
Results of VOR Gains and Asymmetry in Different Groups
Significant difference was found in every VOR gain asymmetry between the VN group and NC group (*p<0.001, Table 1). Significant difference was found in every VOR gain asymmetry between the VN group and AV group (***p<0.001, Table 1). A statistical difference was found in every VOR gain asymmetry between the AV and the NC groups (***p<0.001, Table 1), with close average values, but it was not enough to distinguish abnormal results from artificial error. Except for one case that could not be distinguished, 22 cases involving horizontal SCC function loss were defined as superior vestibular nerve dysfunction, seven cases involving posterior SCC function loss were defined as inferior vestibular nerve dysfunction and three cases involving both were analyzed separately. Significant differences in the affected side of lateral VOR gains were observed between the superior vestibular nerve and AV group, and NC group (p<0.01). Significant differences in the affected side of posterior VOR gains were observed between the inferior vestibular nerve and AV group, and NC group (p<0.01). Despite significant differences in the affected side of VOR, gains both in horizontal and vertical were observed in the whole VN group, the absolute value of vertical VOR gain is not as accurate and stable as VOR gain asymmetry for the artificial error reason.
Sensitivity and Specificity of vHIT in the Diagnosis of VN
According to the previous literature and the results of our repeated tests, 20% or more in horizontal or 25% or more in vertical VOR gain asymmetry were defined as positive and less than 20% horizontal or less than 25% vertical VOR gain asymmetry as negative. The VOR gain asymmetry in VN group were calculated based on affected lateral/intact lateral, affected anterior/intact posterior and affected posterior/intact anterior. Positive results were found in 21 cases with horizontal VOR gain asymmetry, five cases with vertical VOR gain asymmetry, and three in both. Negative results were found in four cases of VN. The sensitivity of vHIT was 87.9% and the specificity was 94.8% in differentiating VN from normals and other acute vertigo; positive predictive value was 85.3% and negative predictive value was 95.8%. The receiver operating characteristic curve is shown in Figure 1.

Figure 1 The receiver operating characteristic curve of sensitivity and specificity of VOR gain asymmetry. Area, 0.943±0.031 (95% confidence interval, 0.882-1.000).
Discussion
VN is an acute unilateral peripheral vestibular disease without other dysfunctions or signs of brain stem involvement. Currently, clinical diagnosis of VN is based on exclusion. Based on our results, we conclude that vHIT is a useful tool that can be used to detect VN patients in the acute phase in an emergency department. We found the VOR gain asymmetry values were significantly different from other AV patients and normal controls, with a high sensitivity (87.9%) and specificity (94.8%), the area under the receiver operating characteristic curve was 0.943, this indicated that vHIT might be a reliable method for diagnosis of VN. Compared with bedside HIT, vHIT requires a relatively smaller head impulse movement and it can be widely used for clinical assessment in the elderly and children, particularly, for bedside examination.Reference Weber, MacDougall and Halmagyi 1 - Reference Armato, Ferri, Pinzani and Ulmer 4 , Reference Agrawal, Schubert and Migliaccio 11 , Reference Wolter, Gordon and Papsin 12 Supportive evidence using a larger sample size may alter the approach to early clinical diagnosis of VN in future.
The vHIT examination to test VOR in three pairs of SSC was not long. Because it was easy and quick for operation, the researchers and doctors expected it could be widely used in the clinic. Comparing vHIT and caloric testing in the diagnosis of peripheral vestibular disease, Bartolomeo et al.Reference Bartolomeo, Biboulet and Pierre 13 found that the sensitivity was 68.84% and the specificity was 100% at the caloric testing value of 30%. McCaslin et al.Reference McCaslin, Jacobson and Bennett 14 showed the sensitivity was 78% and specificity was 95%. These results showed that vHIT specificity was very high but sensitivity was relatively low. In the present study, we demonstrated that vHIT in acute vertigo was ideal for a sensitive and specific diagnosis of VN, with a high sensitivity of 87.9%. Mahringer et al.Reference Mahringer and Rambold 15 considered that sensitivity at acute phase was 61% and only 33% at nonacute phase. The VN patients in our test were all in acute phase and the average time from onset to testing was only 3.6 days, which increased the positive results and might enhance the sensitivity of vHIT. Another reason was that all subjects were screened for hearing. Some of the peripheral vestibular disorders with hearing impairment were included in the previous study, for example, Ménière’s disease might have normal, increased, or decreased VOR,Reference Manzari, Burgess and MacDougall 16 - Reference Zulueta-Santos, Lujan and Manrique-Huarte 18 which would lead to a significant increase in false-positive results. A minority of patients with VN may have high-frequency hearing damageReference Rahko and Karma 19 with only a slight decrease of 16 to 24 dB. Currently, VN is not considered involved in damage to the cochlear nerve. We believe PTA screening is necessary for patients with suspicious hearing impairment, which will significantly increase the objective and specificity of diagnosis. Three negative results of our tests actually approached the cutoff of asymmetry for the diagnosis. In one case, the horizontal asymmetry was 18%. Vertical asymmetry in the remaining two cases was 19% and 21%, respectively; this indicated that the results were close to the cutoff needed to be carefully treated based on clinical features. In another case, the VOR gain asymmetry from three matched pairs was 2% and symptoms of the patient disappeared quickly, although we did the test on the second day. So far, the specificity of vHIT in differential diagnosis of vertebrobasilar stroke in acute vestibular syndrome was highly evaluated, its accuracy rate of vHIT could reach 91% to 100%.Reference Newman-Toker, Saber Tehrani and Mantokoudis 3 , Reference Newman-Toker, Kattah and Alvernia 20 , Reference Mantokoudis, Tehrani and Wozniak 21 Our results contained five patients with vertebrobasilar stroke and their VOR results were all negative; however, they must be judged based on clinical features rather than relying on the vHIT results merely. The VOR gain asymmetry between 20% and 30% is still controversial until now.Reference Newman-Toker, Saber Tehrani and Mantokoudis 3
In contrast to any previous vestibular function tests, vHIT enables the location of vestibular damage and detection of vertical SCC function.Reference Halmagyi, Aw and Cremer 22 , Reference MacDougall, McGarvie and Halmagyi 23 It has traditionally been thought that VN involves the superior vestibular nerve only.Reference Fetter and Dichgans 24 Results of the scleral search coil technique found that VN can also involve the inferior vestibular nerve alone;Reference Aw, Fetter and Cremer 25 therefore, acute peripheral vestibular damage can be divided into three types, which can also be tested by vHIT: (1) horizontal SCC functional loss or horizontal and anterior SCC functional loss; (2) horizontal, anterior, and posterior SCC functional loss; and (3) simple posterior SCC function loss. Halmagyi et al.Reference Halmagyi, Aw and Karlberg 26 reported selective inferior VN patients, whereas horizontal SCC functional loss was not essential for diagnosis of VN. Inferior VN can detect posterior SCC functional loss, which is detected as normal function by caloric testing.Reference Monstad, Okstad and Mygland 27 , Reference Taylor, McGarvie and Reid 28 We summarized our positive results: 15 suffered horizontal SCC functional loss only (Figure 2A), six suffered both horizontal and anterior SCC functional loss (Figure 2B), five involved posterior SCC functional loss (Figure 2C), and three involved the whole SCC (Figure 2D). We believe that increased application of vHIT and data acquisition will facilitate further investigation of the mechanisms of VN. The absolute value of vertical VOR gain is affected greatly by systematic errors, such as patient compliance according to our repeated tests; it might move up or down in pairs in vertical SCC, whereas the VOR gain asymmetry was relatively stable and accurate. To reduce artificial errors, the velocities were mostly controlled below 50° to 200°/second in our research. The lower velocities, especially on vertical SCC, could lose a slight true positive; therefore, suspicious results around cutoff might need to be retested. We look forward more researchers undertaking more tests to explore this issue.

Figure 2 vHIT results in different types of VN patients. (A. Superior VN, patient 1. this showed a significantly reduced VOR gain in the right lateral SCC compared with left lateral SCC. The asymmetry was 50% between the affected and the intact side. (B) Superior VN, patient 2. It showed a significantly reduced VOR gain in the right lateral and anterior SCC compared with left lateral and posterior SCC; the asymmetries were 39% and 29%, respectively. (C) Inferior VN, patient 3. It showed a significantly reduced VOR gain in left posterior SCC compared with right anterior SCC, with an asymmetry of 34%. (D) VN patient 4 with both the superior and inferior vestibular nerve involvement. This showed a significantly reduced VOR gain in all left SCCs compared with the right side; the asymmetry was, respectively, 64%, 34%, and 50%. In a Hex plot of mean gain (in the middle of the image), red=mean gain<unilateral cutoff (0.8 in lateral SCC or <0.7 in anterior or posterior SCC), green=mean gain between unilateral cutoff and 1.2. In the VOR analysis, red=saccade, green=VOR, blue or purple=head movement.
Another advantage of vHIT is that it could record catch-up saccades sensitively, which indicates damages associated with high-frequency vestibular function. In contrast to the low levels of physiological saccade amplitude, the abnormal catch-up saccades not only showed a high amplitude peak speed, but also an increase in speeding head impulse; the positive rate of abnormal catch-up saccades markedly increases in patients with vestibular function impairment.Reference Weber, Aw, Todd, McGarvie, Curthoys and Halmagyi 29 , Reference Blodow, Pannasch and Walther 30 All VN patients in our study were observed with high amplitude catch-up saccades; 24% patients in the AV group also recorded continuous catch-up saccades, with mostly a lower peak. Another interesting point of the results was that saccades were usually recorded in lateral image rather than vertical image, even if the vertical canals were affected heavily. Although some researchers have tried to quantitate saccades,Reference Chen, Todd and Halmagyi 31 it has been difficult to determine the standard to distinguish the results. The positive rate also increased with degeneration factor in the elderly;Reference Agrawal, Schubert and Migliaccio 11 therefore, the role of catch-up saccades in vestibular dysfunction still needs to be investigated in further studies.
In conclusion, this study has shown that vHIT is a simple, quick, and safe test that can mensurate VOR gains in all six semicircular canals quantitatively. The VOR gain asymmetry was relatively more accurate than absolute value of VOR gain, especially in the vertical. It displays good sensitivity and specificity in the early diagnosis of VN associated with acute vertigo without hearing impairment. The results around the cutoff of asymmetry should be treated cautiously.
Acknowledgments and Funding
The study was supported by the Zhejiang Medical Science and Technology Project Foundation (2014KYB136 and 2016KYB264) and the Zhejiang Province Natural Science Foundation (LY15H090004).
Disclosures
QG reports grants from Zhejiang Medical Science and Technology Project Foundation (2016KYB264) outside the submitted work; LZ reports grants from Zhejiang Medical Science and Technology Project Foundation (2014KYB136) and from Zhejiang Province Natural Science Foundation (LY15H090004) outside the submitted work. The remaining authors have nothing to disclose.
Statement of Authorship
XH contributed to design of the study; QG performed the study and executed all the video head impulse tests; ZC analyzed the data; LZ, WH, PL, and DZ evaluated and treated the patients; LZ and YY performed the analysis with constructive discussions.





